Cost-effectiveness of influenza vaccination in prior pneumonia patients in Israel
- PMID: 24930716
- PMCID: PMC4077912
- DOI: 10.1016/j.vaccine.2014.05.015
Cost-effectiveness of influenza vaccination in prior pneumonia patients in Israel
Abstract
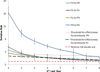
Pneumonia is a common complication of influenza infection, and accounts for the majority of influenza mortality. Both the WHO and the Ministry of Health in Israel prioritize seasonal influenza vaccination primarily on the basis of age and specific co-morbidities. Here we consider whether the targeting of individuals previously infected with pneumonia for influenza vaccination would be a cost-effective addition to the current policy. We performed a retrospective cohort data analysis of 163,990 cases of pneumonia hospitalizations and 1,305,223 cases of outpatient pneumonia from 2004 to 2012, capturing more than 54% of the Israeli population. Our findings demonstrate that patients infected with pneumonia in the year prior had a substantially higher risk of becoming infected with pneumonia in subsequent years (relative risk >2.34, p<0.01). Results indicated that the benefit of targeting for influenza vaccination patients hospitalized with pneumonia in prior year would be cost-saving regardless of age. Complementing the current policy with the targeting of prior pneumonia patients would require vaccination of only a further 2.3% of the Israeli population to save additional 204-407 quality-adjusted life years (QALYs) annually at a mean price of 58-1056 USD/QALY saved. Global uncertainty analysis demonstrates that the cost-effectiveness of adding this policy is robust over a vast range of conditions. As prior pneumonia patients are currently not prioritized for influenza vaccination in Israel, nor elsewhere, this study suggests a novel supplement of current policies to improve cost-effectiveness of influenza vaccination. Future studies should use case-control study to further evaluate the effectiveness of vaccination in prior pneumonia patients.
Keywords: Cost-effectiveness; Influenza and pneumonia; Influenza vaccination.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Grohskopf L, Shay DK, Shimabukuro T, Sokolow L, Keitel W, Bresee JS, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2013–2014. 2013
-
- Nichol KL, D NJ, Nelson DB, Mullooly JP, Hak E. Effectiveness of Influenza Vaccine in the Community-Dwelling Elderly. N Engl J Med. 2007;357:1373–1138. - PubMed
-
- Stephenson I, Hayden F, Osterhaus A, Howard W, Pervikov Y, Palkonyay L, et al. Report of the fourth meeting on"Influenza vaccines that induce broad spectrum and long-lasting immune responses”, World Health Organization and Wellcome Trust, London, United Kingdom, 9–10 November 2009. Vaccine. 2010;28:3875–3882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

