Inhibition of apoptosis in human induced pluripotent stem cells during expansion in a defined culture using angiopoietin-1 derived peptide QHREDGS
- PMID: 24930852
- PMCID: PMC4117357
- DOI: 10.1016/j.biomaterials.2014.05.018
Inhibition of apoptosis in human induced pluripotent stem cells during expansion in a defined culture using angiopoietin-1 derived peptide QHREDGS
Abstract
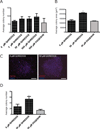
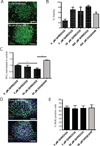
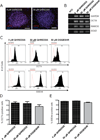
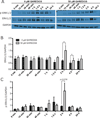
Adhesion molecule signaling is critical to human pluripotent stem cell (hPSC) survival, self-renewal, and differentiation. Thus, hPSCs are grown as clumps of cells on feeder cell layers or poorly defined extracellular matrices such as Matrigel. We sought to define a small molecule that would initiate adhesion-based signaling to serve as a basis for a defined substrate for hPSC culture. Soluble angiopoeitin-1 (Ang-1)-derived peptide QHREDGS added to defined serum-free media increased hPSC colony cell number and size during long- and short-term culture when grown on feeder cell layers or Matrigel, i.e. on standard substrates, without affecting hPSC morphology, growth rate or the ability to differentiate into multiple lineages both in vitro and in vivo. Importantly, QHREDGS treatment decreased hPSC apoptosis during routine passaging and single-cell dissociation. Mechanistically, the interaction of QHREDGS with β1-integrins increased expression of integrin-linked kinase (ILK), increased expression and activation of extracellular signal-regulated kinases 1/2 (ERK1/2), and decreased caspase-3/7 activity. QHREDGS immobilization to polyethylene glycol hydrogels significantly increased cell adhesion in a dose-dependent manner. We propose QHREDGS as a small molecule inhibitor of hPSC apoptosis and the basis of an affordable defined substrate for hPSC maintenance.
Keywords: Adhesion; Angiopoietin-1; Apoptosis; Human pluripotent stem cells; Integrins; QHREDGS.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Photocrosslinkable chitosan modified with angiopoietin-1 peptide, QHREDGS, promotes survival of neonatal rat heart cells.J Biomed Mater Res A. 2010 Oct;95(1):105-17. doi: 10.1002/jbm.a.32808. J Biomed Mater Res A. 2010. PMID: 20540095
-
Wnt signaling orchestration with a small molecule DYRK inhibitor provides long-term xeno-free human pluripotent cell expansion.Stem Cells Transl Med. 2012 Jan;1(1):18-28. doi: 10.5966/sctm.2011-0033. Epub 2011 Dec 7. Stem Cells Transl Med. 2012. PMID: 23197636 Free PMC article.
-
Synergistic effect of medium, matrix, and exogenous factors on the adhesion and growth of human pluripotent stem cells under defined, xeno-free conditions.Stem Cells Dev. 2012 Jul 20;21(11):2036-48. doi: 10.1089/scd.2011.0489. Epub 2012 Jan 26. Stem Cells Dev. 2012. PMID: 22149941
-
Concise review: The evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings.Stem Cells. 2013 Jan;31(1):1-7. doi: 10.1002/stem.1260. Stem Cells. 2013. PMID: 23081828 Free PMC article. Review.
-
Biological Effects of Culture Substrates on Human Pluripotent Stem Cells.Stem Cells Int. 2016;2016:5380560. doi: 10.1155/2016/5380560. Epub 2016 Aug 30. Stem Cells Int. 2016. PMID: 27656216 Free PMC article. Review.
Cited by
-
Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):E5792-E5801. doi: 10.1073/pnas.1612277113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647919 Free PMC article.
-
Delivery of MSCs with a Hybrid β-Sheet Peptide Hydrogel Consisting IGF-1C Domain and D-Form Peptide for Acute Kidney Injury Therapy.Int J Nanomedicine. 2020 Jun 17;15:4311-4324. doi: 10.2147/IJN.S254635. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32606679 Free PMC article.
-
3D-bioprinted peptide coupling patches for wound healing.Mater Today Bio. 2021 Dec 11;13:100188. doi: 10.1016/j.mtbio.2021.100188. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34977527 Free PMC article.
-
Application of an instructive hydrogel accelerates re-epithelialization of xenografted human skin wounds.Sci Rep. 2022 Aug 20;12(1):14233. doi: 10.1038/s41598-022-18204-w. Sci Rep. 2022. PMID: 35987767 Free PMC article.
-
Design of Recombinant Spider Silk Proteins for Cell Type Specific Binding.Adv Healthc Mater. 2023 Apr;12(9):e2202660. doi: 10.1002/adhm.202202660. Epub 2023 Jan 13. Adv Healthc Mater. 2023. PMID: 36565209 Free PMC article.
References
-
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. - PubMed
-
- Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

