Activation of the APC/C ubiquitin ligase by enhanced E2 efficiency
- PMID: 24930963
- PMCID: PMC4090254
- DOI: 10.1016/j.cub.2014.05.052
Activation of the APC/C ubiquitin ligase by enhanced E2 efficiency
Abstract
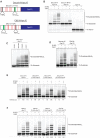
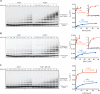
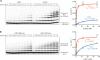
The anaphase-promoting complex/cyclosome (APC/C) is a protein-ubiquitin ligase (E3) that initiates the final events of mitosis by catalyzing the ubiquitination and proteasomal destruction of securin, cyclins, and other substrates [1, 2]. Like other members of the RING family of E3s [3, 4], the APC/C catalyzes direct ubiquitin transfer from an E2-ubiquitin conjugate (E2-Ub) to lysine residues on the protein substrate. The APC/C is activated at specific cell-cycle stages by association with an activator subunit, Cdc20 or Cdh1, which provides binding sites for specific substrate sequence motifs, or degrons. Activator might also stimulate catalytic activity [5, 6], but the underlying mechanisms are not known. Here, we dissected activator function using an artificial fusion substrate in which the N-terminal region of securin was linked to an APC/C core subunit. This fusion substrate bound tightly to the APC/C and was ubiquitinated at a low rate in the absence of activator. Ubiquitination of this substrate was stimulated by activator, due primarily to a dramatic stimulation of E2 sensitivity (Km) and catalytic rate (kcat), which together resulted in a 670-fold stimulation of kcat/Km. Thus, activator is not simply a substrate adaptor, but also enhances catalysis by promoting a more efficient interaction with the E2-Ub. Interestingly, full E2 stimulation required activator interaction with degron motifs on the substrate. We conclude that formation of a complete APC/C-activator-substrate complex leads to a major enhancement of E2 efficiency, providing an unusual substrate-assisted catalytic mechanism that limits efficient ubiquitin transfer to specific substrates.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C). Q. Rev. Biophys. 2011;44:153–190. - PubMed
-
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. - PubMed
-
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 2006;8:607–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

