Caspase-8-mediated PAR-4 cleavage is required for TNFα-induced apoptosis
- PMID: 24931006
- PMCID: PMC4102785
- DOI: 10.18632/oncotarget.1634
Caspase-8-mediated PAR-4 cleavage is required for TNFα-induced apoptosis
Abstract
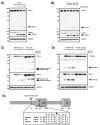
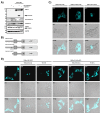
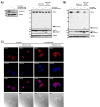
The tumor suppressor protein prostate apoptosis response-4 (PAR-4) is silenced in a subset of human cancers and its down-regulation serves as a mechanism for cancer cell survival following chemotherapy. PAR-4 re-expression selectively causes apoptosis in cancer cells but how its pro-apoptotic functions are controlled and executed precisely is currently unknown. We demonstrate here that UV-induced apoptosis results in a rapid caspase-dependent PAR-4 cleavage at EEPD131G, a sequence that was preferentially recognized by caspase-8. To investigate the effect on cell growth for this cleavage event we established stable cell lines that express wild-type-PAR-4 or the caspase cleavage resistant mutant PAR-4 D131G under the control of a doxycycline-inducible promoter. Induction of the wild-type protein but not the mutant interfered with cell proliferation, predominantly through induction of apoptosis. We further demonstrate that TNFα-induced apoptosis leads to caspase-8-dependent PAR-4-cleavage followed by nuclear accumulation of the C-terminal PAR-4 (132-340) fragment, which then induces apoptosis. Taken together, our results indicate that the mechanism by which PAR-4 orchestrates the apoptotic process requires cleavage by caspase-8.
Figures


References
-
- Sells SF, Wood DP, Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM. Commonality of the gene programs induced by effectors of apoptosis in androgen- dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. - PubMed
-
- Cook J, Krishnan S, Ananch S, Sells SF, Shi Y, Walther MM, Linehan WM, Sukhatme VP, Weinstein MH, Rangnekar VM. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene. 1999;18:1205–1208. - PubMed
-
- Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, Hardisson D, Diaz-Meco MT, Moscat J, Serrano M, Palacios J. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

