Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor
- PMID: 24931123
- PMCID: PMC4101812
- DOI: 10.1016/j.immuni.2014.05.007
Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor
Abstract
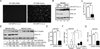
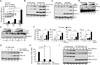
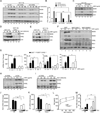
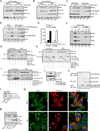
Virus infection is sensed in the cytoplasm by retinoic acid-inducible gene I (RIG-I, also known as DDX58), which requires RNA and polyubiquitin binding to induce type I interferon (IFN) and activate cellular innate immunity. We show that the human IFN-inducible oligoadenylate synthetases-like (OASL) protein has antiviral activity and mediates RIG-I activation by mimicking polyubiquitin. Loss of OASL expression reduced RIG-I signaling and enhanced virus replication in human cells. Conversely, OASL expression suppressed replication of a number of viruses in a RIG-I-dependent manner and enhanced RIG-I-mediated IFN induction. OASL interacted and colocalized with RIG-I, and through its C-terminal ubiquitin-like domain specifically enhanced RIG-I signaling. Bone-marrow-derived macrophages from mice deficient for Oasl2 showed that among the two mouse orthologs of human OASL, Oasl2 is functionally similar to human OASL. Our findings show a mechanism by which human OASL contributes to host antiviral responses by enhancing RIG-I activation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

