Maturation of membrane properties of neurons in the rat deep cerebellar nuclei
- PMID: 24931427
- PMCID: PMC4211972
- DOI: 10.1002/dneu.22203
Maturation of membrane properties of neurons in the rat deep cerebellar nuclei
Abstract
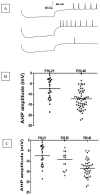
Patch clamp recordings of neurons in the adult rat deep cerebellar nuclei have been limited by the availability of viable brain slices. Using a new slicing technique, this study was designed to explore the maturation of membrane properties of neurons in the deep cerebellar nuclei (DCN)-an area involved in rat eyeblink conditioning. Compared to whole-cell current-clamp recordings in DCN in rat pups at postnatal day 16 (P16) to P21, recordings from weanling rats at P22-P40 revealed a number of significant changes including an increase in the amplitude of the afterhyperpolarization (AHP)-an index of membrane excitability which has been shown to be important for eyeblink conditioning-a prolonged interval between the first and second evoked action potential, and an increase in AHP amplitude for hyperpolarization-induced rebound spikes. This is the first report of developmental changes in membrane properties of DCN which may contribute to the ontogeny of eyeblink conditioning in the rat.
Keywords: after-hyperpolarization; cerebellar nuclear neurons; developmental changes; maturation; membrane property.
© 2014 Wiley Periodicals, Inc.
Figures


Similar articles
-
Changes in membrane properties of rat deep cerebellar nuclear projection neurons during acquisition of eyeblink conditioning.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):E9419-E9428. doi: 10.1073/pnas.1808539115. Epub 2018 Aug 28. Proc Natl Acad Sci U S A. 2018. PMID: 30154170 Free PMC article.
-
Disruption of rat deep cerebellar perineuronal net alters eyeblink conditioning and neuronal electrophysiology.Neurobiol Learn Mem. 2021 Jan;177:107358. doi: 10.1016/j.nlm.2020.107358. Epub 2020 Dec 4. Neurobiol Learn Mem. 2021. PMID: 33285318 Free PMC article.
-
Changes in cerebellar intrinsic neuronal excitability and synaptic plasticity result from eyeblink conditioning.Neurobiol Learn Mem. 2019 Dec;166:107094. doi: 10.1016/j.nlm.2019.107094. Epub 2019 Sep 19. Neurobiol Learn Mem. 2019. PMID: 31542329 Free PMC article. Review.
-
Analysis of distinct short and prolonged components in rebound spiking of deep cerebellar nucleus neurons.Eur J Neurosci. 2010 Nov;32(10):1646-57. doi: 10.1111/j.1460-9568.2010.07408.x. Epub 2010 Oct 8. Eur J Neurosci. 2010. PMID: 21039958 Free PMC article.
-
Rebound discharge in deep cerebellar nuclear neurons in vitro.Cerebellum. 2010 Sep;9(3):352-74. doi: 10.1007/s12311-010-0168-7. Cerebellum. 2010. PMID: 20396983 Free PMC article. Review.
Cited by
-
Dietary cholesterol concentration affects synaptic plasticity and dendrite spine morphology of rabbit hippocampal neurons.Brain Res. 2015 Oct 5;1622:350-60. doi: 10.1016/j.brainres.2015.06.049. Epub 2015 Jul 16. Brain Res. 2015. PMID: 26188241 Free PMC article.
-
The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning.Biology (Basel). 2024 Mar 21;13(3):200. doi: 10.3390/biology13030200. Biology (Basel). 2024. PMID: 38534469 Free PMC article. Review.
-
Changes in membrane properties of rat deep cerebellar nuclear projection neurons during acquisition of eyeblink conditioning.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):E9419-E9428. doi: 10.1073/pnas.1808539115. Epub 2018 Aug 28. Proc Natl Acad Sci U S A. 2018. PMID: 30154170 Free PMC article.
-
Disruption of rat deep cerebellar perineuronal net alters eyeblink conditioning and neuronal electrophysiology.Neurobiol Learn Mem. 2021 Jan;177:107358. doi: 10.1016/j.nlm.2020.107358. Epub 2020 Dec 4. Neurobiol Learn Mem. 2021. PMID: 33285318 Free PMC article.
-
Changes in cerebellar intrinsic neuronal excitability and synaptic plasticity result from eyeblink conditioning.Neurobiol Learn Mem. 2019 Dec;166:107094. doi: 10.1016/j.nlm.2019.107094. Epub 2019 Sep 19. Neurobiol Learn Mem. 2019. PMID: 31542329 Free PMC article. Review.
References
-
- Aizenman CD, Huang EJ, Linden DJ. Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J Neurophysiol. 2003;89:1738–1747. - PubMed
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. - PubMed
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

