Measurement of rivaroxaban and apixaban in serum samples of patients
- PMID: 24931429
- PMCID: PMC4143952
- DOI: 10.1111/eci.12291
Measurement of rivaroxaban and apixaban in serum samples of patients
Abstract
Background: The determination of rivaroxaban and apixaban from serum samples of patients may be beneficial in specific clinical situations when additional blood sampling for plasma and thus the determination of factor Xa activity is not feasible or results are not plausible.
Materials and methods: The primary aim of this study was to compare the concentrations of rivaroxaban and apixaban in serum with those measured in plasma. Secondary aims were the performance of three different chromogenic methods and concentrations in patients on treatment with rivaroxaban 10 mg od (n = 124) or 20 mg od (n = 94) or apixaban 5 mg bid (n = 52) measured at different time.
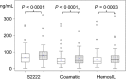
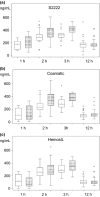
Results: Concentrations of rivaroxaban and apixaban in serum were about 20-25% higher compared with plasma samples with a high correlation (r = 0·79775-0·94662) using all assays (all P < 0·0001). The intraclass correlation coefficients were about 0·90 for rivaroxaban and 0·55 for apixaban. Mean rivaroxaban concentrations were higher at 2 and 3 h compared with 1 and 12 h after administration measured from plasma and serum samples (all P-values < 0·05) and were not different between 1 vs. 12 h (plasma and serum).
Conclusions: The results indicate that rivaroxaban and apixaban concentrations can be determined specifically from serum samples.
Keywords: Apixaban; chromogenic substrate methods; direct oral anticoagulants; plasma; rivaroxaban; serum.
© 2014 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Figures

Similar articles
-
Evaluation of global laboratory methods and establishing on-therapy ranges for monitoring apixaban and rivaroxaban: Experience at a single institution.J Clin Lab Anal. 2019 Jun;33(5):e22869. doi: 10.1002/jcla.22869. Epub 2019 Mar 12. J Clin Lab Anal. 2019. PMID: 30860622 Free PMC article.
-
Anti-Xa Oral Anticoagulant Plasma Concentration Assay in Real Life: Rivaroxaban and Apixaban Quantification in Emergency With LMWH Calibrator.Ann Pharmacother. 2019 Apr;53(4):341-347. doi: 10.1177/1060028018811657. Epub 2018 Oct 31. Ann Pharmacother. 2019. PMID: 30378443
-
Measuring the activity of apixaban and rivaroxaban with rotational thrombelastometry.Thromb Res. 2014 Oct;134(4):918-23. doi: 10.1016/j.thromres.2014.08.006. Epub 2014 Aug 19. Thromb Res. 2014. PMID: 25179518
-
Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants.J Am Coll Cardiol. 2014 Sep 16;64(11):1128-39. doi: 10.1016/j.jacc.2014.05.065. J Am Coll Cardiol. 2014. PMID: 25212648 Free PMC article. Review.
-
Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment.Clin Pharmacokinet. 2013 Apr;52(4):243-54. doi: 10.1007/s40262-013-0034-0. Clin Pharmacokinet. 2013. PMID: 23389892 Review.
Cited by
-
Scalable manufacture of a disposable, storage-stable eight-channel microfluidic device for rapid testing of platelet, coagulation, and drug function under whole blood flow.Biomicrofluidics. 2020 Sep 29;14(5):054103. doi: 10.1063/5.0023312. eCollection 2020 Sep. Biomicrofluidics. 2020. PMID: 33014235 Free PMC article.
-
Rivaroxaban and apixaban in orthopaedics: is there a difference in their plasma concentrations and anticoagulant effects?Blood Coagul Fibrinolysis. 2015 Dec;26(8):925-33. doi: 10.1097/MBC.0000000000000371. Blood Coagul Fibrinolysis. 2015. PMID: 26258673 Free PMC article.
-
Non-VKA Oral Anticoagulants: Accurate Measurement of Plasma Drug Concentrations.Biomed Res Int. 2015;2015:345138. doi: 10.1155/2015/345138. Epub 2015 May 19. Biomed Res Int. 2015. PMID: 26090400 Free PMC article. Review.
-
Rivaroxaban Effects Illustrate the Underestimated Importance of Activated Platelets in Thrombin Generation Assessed by Calibrated Automated Thrombography.J Clin Med. 2019 Nov 15;8(11):1990. doi: 10.3390/jcm8111990. J Clin Med. 2019. PMID: 31731710 Free PMC article.
References
-
- Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JW. Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement: a systematic review. Ann Intern Med. 2013;159:275–84. - PubMed
-
- Kansal AR, Sharma M, Bradley-Kennedy C, Clemens A, Monz BU, Peng S, et al. Dabigatran versus rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thromb Haemost. 2012;108:672–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

