Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity
- PMID: 24931705
- PMCID: PMC4223010
- DOI: 10.1016/j.biopsych.2014.04.014
Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity
Abstract
Background: One of the most novel and exciting findings in major depressive disorder research over the last decade is the discovery of the fast-acting and long-lasting antidepressant effects of ketamine. Indeed, the therapeutic effects of classic antidepressants, such as selective serotonin reuptake inhibitors, require a month or longer to be expressed, with about a third of major depressive disorder patients resistant to treatment. Clinical studies have shown that a low dose of ketamine exhibits fast-acting relatively sustained antidepressant action, even in treatment-resistant patients. However, the mechanisms of ketamine action at a systems level remain unclear.
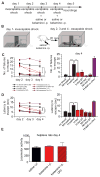
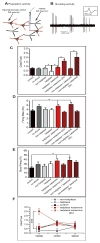
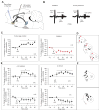
Methods: Wistar-Kyoto rats were exposed to inescapable, uncontrollable footshocks. To evaluate learned helplessness behavior, we used an active avoidance task in a shuttle box equipped with an electrical grid floor. After helplessness assessment, we performed in vivo electrophysiological recordings first from ventral tegmental area dopaminergic (DA) neurons and second from accumbens neurons responsive to fimbria stimulation. Ketamine was injected and tested on helpless behavior and electrophysiological recordings.
Results: We show that ketamine is able to restore the integrity of a network by acting on the DA system and restoring synaptic dysfunction observed in stress-induced depression. We show that part of the antidepressant effect of ketamine is via the DA system. Indeed, injection of ketamine restores a decreased dopamine neuron population activity, as well as synaptic plasticity (long-term potentiation) in the hippocampus-accumbens pathway, via, in part, activation of D1 receptors.
Conclusions: This work provides a unique systems perspective on the mechanisms of ketamine on a disrupted limbic system.
Keywords: Dopamine; ketamine; learned helplessness; nucleus accumbens; synaptic plasticity; ventral tegmental area.
Copyright © 2014 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
-
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. - PubMed
-
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

