Pilot study of nasal expiratory positive airway pressure devices for the treatment of childhood obstructive sleep apnea syndrome
- PMID: 24932147
- PMCID: PMC4031408
- DOI: 10.5664/jcsm.3796
Pilot study of nasal expiratory positive airway pressure devices for the treatment of childhood obstructive sleep apnea syndrome
Abstract
Study objectives: Alternative therapies for childhood obstructive sleep apnea syndrome (OSAS) are needed as OSAS may persist despite adenotonsillectomy, and continuous positive airway pressure (CPAP) adherence is low. Nasal expiratory positive airway pressure (NEPAP) devices have not been studied in children. We hypothesized that NEPAP would result in polysomnographic improvement. Further, we aimed to determine NEPAP adherence, effects on sleepiness, behavior, and quality of life.
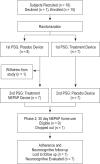
Methods: A randomized, double-blind, placebo-controlled, crossover pilot study was performed. CPAP candidates, 8-16 years old, underwent NEPAP and placebo polysomnograms. Subjects with ≥ 50% reduction in the apnea hypopnea index (AHI) from placebo to NEPAP night or AHI < 5/h on NEPAP night wore NEPAP at home for 30 days. Adherence was assessed by daily phone calls/emails and collecting used devices.
Results: Fourteen subjects (age 13.4 ± 1.9 years, BMI z-scores 2.2 ± 1 [mean ± SD]) were studied. There was significant improvement in the obstructive apnea index with NEPAP vs. placebo: 0.6 (0-21.1)/h vs. 4.2 (0-41.9)/h (median [range], p = 0.010) and trends for improvement in other polysomnographic parameters. However, responses were variable, with 3 subjects not improving and 2 worsening. Older children and those with less hypercapnia had a better response. Eight subjects were sent home with devices; one was lost to follow-up, and adherence in the remainder was 83% of nights; these subjects had a significant improvement in sleepiness and quality of life.
Conclusions: NEPAP devices are a potential alternative therapy for OSAS in a small subset of children. Due to variability in individual responses, efficacy of NEPAP should be evaluated with polysomnography.
Clinical trial registration: www.clinicaltrials.gov, identifier: NCT01768065.
Keywords: nasal expiratory positive airway pressure; obstructive sleep apnea syndrome; pediatrics.
Figures


References
-
- Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. - PubMed
-
- Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

