Guidelines for therapeutic drug monitoring of vancomycin: a systematic review
- PMID: 24932495
- PMCID: PMC4059638
- DOI: 10.1371/journal.pone.0099044
Guidelines for therapeutic drug monitoring of vancomycin: a systematic review
Abstract
Background and objective: Despite the availability of clinical practice guidelines (CPGs) for therapeutic drug monitoring (TDM) of vancomycin, vancomycin serum concentrations still do not reach therapeutic concentrations in many patients. Thus, we sought to systematically review the quality and consistency of recommendations for an international cohort of CPGs regarding vancomycin TDM.
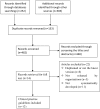
Methods: PubMed, Embase, guidelines' websites and Google were searched for CPGs for vancomycin TDM. Two independent assessors rated the quality of each CPG using the Appraisal of Guidelines for Research & Evaluation II (AGREEII) instrument and data were independently extracted.
Results: Twelve guidelines were evaluated and the overall quality of guidelines for vancomycin TDM was moderate. The highest score was recorded in the domain of clarity of presentation, and the lowest score was recorded in the domain of rigor of development and stakeholder involvement. The specific recommendations for vancomycin TDM were moderately consistent and guidelines varied in trough concentration monitoring, frequency of TDM, and serum concentration targets.
Conclusion: The overall guideline quality for vancomycin TDM was not optimal and effort is needed to improve guideline quality, especially in the domain of rigor of development and stakeholder involvement.
Conflict of interest statement
Figures
References
-
- Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N (2012) Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67: 17–24. - PubMed
-
- Swartling M, Gupta R, Dudas V, Guglielmo BJ (2012) Short term impact of guidelines on vancomycin dosing and therapeutic drug monitoring. Int J Clin Pharm 34: 282–285. - PubMed
-
- Eiland LS, English TM, Eiland EH (2011) Assessment of Vancomycin Dosing and Subsequent Serum Concentrations in Pediatric Patients. Annals of Pharmacotherapy 45: 582–589. - PubMed
-
- De Cock P, Haegeman E, Goerlandt N, Vanhaesebrouck P, Stove V, et al. (2010) Focused Conference Group: P13 - Maximising benefits and minimizing harms from drugs vancomycin dosing and monitoring in children: Compliance with guidelines in a belgian teaching hospital. Basic and Clinical Pharmacology and Toxicology 107: 250–251.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical