Stimulatory interactions between human coronary smooth muscle cells and dendritic cells
- PMID: 24932497
- PMCID: PMC4059651
- DOI: 10.1371/journal.pone.0099652
Stimulatory interactions between human coronary smooth muscle cells and dendritic cells
Abstract
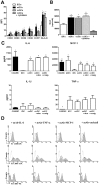
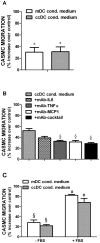
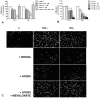
Despite inflammatory and immune mechanisms participating to atherogenesis and dendritic cells (DCs) driving immune and non-immune tissue injury response, the interactions between DCs and vascular smooth muscle cells (VSMCs) possibly relevant to vascular pathology including atherogenesis are still unclear. To address this issue, immature DCs (iDCs) generated from CD14+ cells isolated from healthy donors were matured either with cytokines (mDCs), or co-cultured (ccDCs) with human coronary artery VSMCs (CASMCs) using transwell chambers. Co-culture induced DC immunophenotypical and functional maturation similar to cytokines, as demonstrated by flow cytometry and mixed lymphocyte reaction. In turn, factors from mDCs and ccDCs induced CASMC migration. MCP-1 and TNFα, secreted from DCs, and IL-6 and MCP-1, secreted from CASMCs, were primarily involved. mDCs adhesion to CASMCs was enhanced by CASMC pre-treatment with IFNγ and TNFα ICAM-1 and VCAM-1 were involved, since the expression of specific mRNAs for these molecules increased and adhesion was inhibited by neutralizing antibodies to the counter-receptors CD11c and CD18. Adhesion was also inhibited by CASMC pre-treatment with the HMG-CoA-reductase inhibitor atorvastatin and the PPARγ agonist rosiglitazone, which suggests a further mechanism for the anti-inflammatory action of these drugs. Adhesion of DCs to VSMCs was shown also in vivo in rat carotid 7 to 21 days after crush and incision injury. The findings indicate that DCs and VSMCs can interact with reciprocal stimulation, possibly leading to perpetuate inflammation and vascular wall remodelling, and that the interaction is enhanced by a cytokine-rich inflammatory environment and down-regulated by HMGCoA-reductase inhibitors and PPARγ agonists.
Conflict of interest statement
Figures



References
-
- Libby P (2002) Inflammation in atherosclerosis. Nature 420: 868–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

