Tight coupling of Na+/K+-ATPase with glycolysis demonstrated in permeabilized rat cardiomyocytes
- PMID: 24932585
- PMCID: PMC4059654
- DOI: 10.1371/journal.pone.0099413
Tight coupling of Na+/K+-ATPase with glycolysis demonstrated in permeabilized rat cardiomyocytes
Abstract
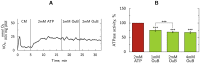
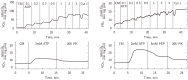
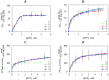
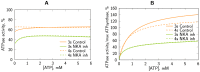
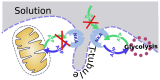
The effective integrated organization of processes in cardiac cells is achieved, in part, by the functional compartmentation of energy transfer processes. Earlier, using permeabilized cardiomyocytes, we demonstrated the existence of tight coupling between some of cardiomyocyte ATPases and glycolysis in rat. In this work, we studied contribution of two membrane ATPases and whether they are coupled to glycolysis--sarcoplasmic reticulum Ca2+ ATPase (SERCA) and plasmalemma Na+/K+-ATPase (NKA). While SERCA activity was minor in this preparation in the absence of calcium, major role of NKA was revealed accounting to ∼30% of the total ATPase activity which demonstrates that permeabilized cell preparation can be used to study this pump. To elucidate the contribution of NKA in the pool of ATPases, a series of kinetic measurements was performed in cells where NKA had been inhibited by 2 mM ouabain. In these cells, we recorded: ADP- and ATP-kinetics of respiration, competition for ADP between mitochondria and pyruvate kinase (PK), ADP-kinetics of endogenous PK, and ATP-kinetics of total ATPases. The experimental data was analyzed using a series of mathematical models with varying compartmentation levels. The results show that NKA is tightly coupled to glycolysis with undetectable flux of ATP between mitochondria and NKA. Such tight coupling of NKA to PK is in line with its increased importance in the pathological states of the heart when the substrate preference shifts to glucose.
Conflict of interest statement
Figures


References
-
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, et al. (2001) Energetic crosstalk between organelles: Architectural integration of energy production and utilization. Circ Res 89: 153–159. - PubMed
-
- Neubauer S (2007) The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151. - PubMed
-
- Vendelin M, Eimre M, Seppet E, Peet N, Andrienko T, et al. (2004) Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol Cell Biochem 256–257: 229–241. - PubMed
-
- Kay L, Nicolay K, Wieringa B, Saks V, Wallimann T (2000) Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J Biol Chem 275: 6937–6944. - PubMed
-
- Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, et al. (2001) Functional complexes of mitochondria with ca,mgatpases of myofibrils and sarcoplasmic reticulum in muscle cells. BBA 1504: 379–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

