GATA4 is essential for bone mineralization via ERα and TGFβ/BMP pathways
- PMID: 24932701
- PMCID: PMC4501475
- DOI: 10.1002/jbmr.2296
GATA4 is essential for bone mineralization via ERα and TGFβ/BMP pathways
Abstract
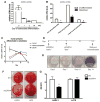
Osteoporosis is a disease characterized by low bone mass, leading to an increased risk of fragility fractures. GATA4 is a zinc-finger transcription factor that is important in several tissues, such as the heart and intestines, and has recently been shown to be a pioneer factor for estrogen receptor alpha (ERα) in osteoblast-like cells. Herein, we demonstrate that GATA4 is necessary for estrogen-mediated transcription and estrogen-independent mineralization in vitro. In vivo deletion of GATA4, driven by Cre-recombinase in osteoblasts, results in perinatal lethality, decreased trabecular bone properties, and abnormal bone development. Microarray analysis revealed GATA4 suppression of TGFβ signaling, necessary for osteoblast progenitor maintenance, and concomitant activation of BMP signaling, necessary for mineralization. Indeed, pSMAD1/5/8 signaling, downstream of BMP signaling, is decreased in the trabecular region of conditional knockout femurs, and pSMAD2/3, downstream of TGFβ signaling, is increased in the same region. Together, these experiments demonstrate the necessity of GATA4 in osteoblasts. Understanding the role of GATA4 to regulate the tissue specificity of estrogen-mediated osteoblast gene regulation and estrogen-independent bone differentiation may help to develop therapies for postmenopausal osteoporosis.
Keywords: ESTROGEN; GATA4; TGF BETA, BMP, OSTEOBLAST.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures




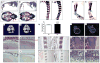


References
-
- United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. Available from: http://www.bone-andjointburden.org/chapter_downloads/index.htm.
-
- Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–30. - PubMed
-
- Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275(50):38949–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

