Control of protein function through optochemical translocation
- PMID: 24933258
- PMCID: PMC4210160
- DOI: 10.1021/sb400192a
Control of protein function through optochemical translocation
Abstract
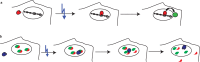
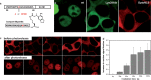
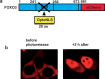
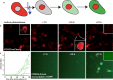
Controlled manipulation of proteins and their function is important in almost all biological disciplines. Here, we demonstrate control of protein activity with light. We present two different applications-light-triggered transcription and light-triggered protease cleavage-both based on the same concept of protein mislocation, followed by optochemically triggered translocation to an active cellular compartment. In our approach, we genetically encode a photocaged lysine into the nuclear localization signal (NLS) of the transcription factor SATB1. This blocks nuclear import of the protein until illumination induces caging group removal and release of the protein into the nucleus. In the first application, prepending this NLS to the transcription factor FOXO3 allows us to optochemically switch on its transcription activity. The second application uses the developed light-activated NLS to control nuclear import of TEV protease and subsequent cleavage of nuclear proteins containing TEV cleavage sites. The small size of the light-controlled NLS (only 20 amino acids) minimizes impact of its insertion on protein function and promises a general approach to a wide range of optochemical applications. Since the light-activated NLS is genetically encoded and optically triggered, it will prove useful to address a variety of problems requiring spatial and temporal control of protein function, for example, in stem-cell, developmental, and cancer biology.
Keywords: nuclear import; optogenetics; photocontrolled TEV-cleavage; photocontrolled transcription; protein control.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

