Adenomatous polyposis coli protein deletion leads to cognitive and autism-like disabilities
- PMID: 24934177
- PMCID: PMC4317257
- DOI: 10.1038/mp.2014.61
Adenomatous polyposis coli protein deletion leads to cognitive and autism-like disabilities
Abstract
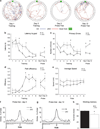
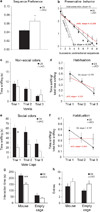
Intellectual disabilities (IDs) and autism spectrum disorders link to human APC inactivating gene mutations. However, little is known about adenomatous polyposis coli's (APC's) role in the mammalian brain. This study is the first direct test of the impact of APC loss on central synapses, cognition and behavior. Using our newly generated APC conditional knock-out (cKO) mouse, we show that deletion of this single gene in forebrain neurons leads to a multisyndromic neurodevelopmental disorder. APC cKO mice, compared with wild-type littermates, exhibit learning and memory impairments, and autistic-like behaviors (increased repetitive behaviors, reduced social interest). To begin to elucidate neuronal changes caused by APC loss, we focused on the hippocampus, a key brain region for cognitive function. APC cKO mice display increased synaptic spine density, and altered synaptic function (increased frequency of miniature excitatory synaptic currents, modestly enhanced long-term potentiation). In addition, we found excessive β-catenin levels and associated changes in canonical Wnt target gene expression and N-cadherin synaptic adhesion complexes, including reduced levels of presenilin1. Our findings identify some novel functional and molecular changes not observed previously in other genetic mutant mouse models of co-morbid cognitive and autistic-like disabilities. This work thereby has important implications for potential therapeutic targets and the impact of their modulation. We provide new insights into molecular perturbations and cell types that are relevant to human ID and autism. In addition, our data elucidate a novel role for APC in the mammalian brain as a hub that links to and regulates synaptic adhesion and signal transduction pathways critical for normal cognition and behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
New molecular insights into cognitive and autistic-like disabilities.Mol Psychiatry. 2014 Oct;19(10):1053. doi: 10.1038/mp.2014.129. Mol Psychiatry. 2014. PMID: 25288258 Free PMC article. No abstract available.
References
-
- Herrera L, Kakati S, Gibas L, Pietrzak E, Sandberg AA. Gardner syndrome in a man with an interstitial deletion of 5q. Am J Med Genet. 1986;25:473–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

