Effects of replacement of factor VIII amino acids Asp519 and Glu665 with Val on plasma survival and efficacy in vivo
- PMID: 24934295
- PMCID: PMC4147049
- DOI: 10.1208/s12248-014-9627-2
Effects of replacement of factor VIII amino acids Asp519 and Glu665 with Val on plasma survival and efficacy in vivo
Abstract
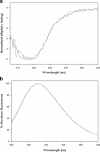
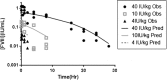
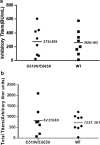
Proteolytic cleavage of factor VIII (FVIII) to activated FVIIIa is required for participation in the coagulation cascade. The A2 domain is no longer covalently bound in the resulting activated heterotrimer and is highly unstable. Aspartic acid (D) 519 and glutamic acid (E) 665 at the A1-A2 and A2-A3 domain interfaces were identified as acidic residues in local hydrophobic pockets. Replacement with hydrophobic valine (V; D519V/E665V) improved the stability and activity of the mutant FVIII over the wild-type (WT) protein in several in vitro assays. In the current study, we examined the impact of mutations on secondary and tertiary structure as well as in vivo stability, pharmacokinetics (PK), efficacy, and immunogenicity in a murine model of Hemophilia A (HA). Biophysical characterization was performed with far-UV circular dichroism (CD) and fluorescence emission studies. PK and efficacy of FVIII was studied following i.v. bolus doses of 4, 10 and 40 IU/kg with chromogenic and tail clip assays. Immunogenicity was measured with the Bethesda assay and ELISA after a series of i.v. injections. Native secondary and tertiary structure was unaltered between variants. PK profiles were similar at higher doses, but at 4 IU/kg plasma survival of D519V/E665V was improved. Hemostasis at low concentrations was improved for the mutant. Immune response was similar between variants. Overall, these results demonstrate that stabilizing mutations in the A2 domain of FVIII can improve HA therapy in vivo.
Figures


Similar articles
-
The combination of Asp519Val/Glu665Val and Lys1813Ala mutations in FVIII markedly increases coagulation potential.Blood Adv. 2024 Aug 13;8(15):3929-3940. doi: 10.1182/bloodadvances.2023012391. Blood Adv. 2024. PMID: 38820442 Free PMC article.
-
Noncovalent stabilization of the factor VIII A2 domain enhances efficacy in hemophilia A mouse vascular injury models.Blood. 2015 Jan 8;125(2):392-8. doi: 10.1182/blood-2014-02-555656. Epub 2014 Oct 20. Blood. 2015. PMID: 25331117 Free PMC article.
-
Soy phosphatidylinositol containing nanoparticle prolongs hemostatic activity of B-domain deleted factor VIII in hemophilia A mice.J Pharm Sci. 2015 Feb;104(2):388-95. doi: 10.1002/jps.23963. Epub 2014 Apr 2. J Pharm Sci. 2015. PMID: 24700333 Free PMC article.
-
Recombinant factor VIII: past, present and future of treatment of hemophilia A.Drugs Today (Barc). 2018 Apr;54(4):269-281. doi: 10.1358/dot.2018.54.4.2800622. Drugs Today (Barc). 2018. PMID: 29869648 Review.
-
The protein structure and effect of factor VIII.Thromb Res. 2007;119(1):1-13. doi: 10.1016/j.thromres.2005.12.015. Epub 2006 Feb 17. Thromb Res. 2007. PMID: 16487577 Review.
Cited by
-
Long-term correction of hemorrhagic diathesis in hemophilia A mice by an AAV-delivered hybrid FVIII composed of the human heavy chain and the rat light chain.Front Med. 2022 Aug;16(4):584-595. doi: 10.1007/s11684-021-0844-7. Epub 2022 Jan 17. Front Med. 2022. PMID: 35038106
References
-
- Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263(13):6352–62. - PubMed
-
- Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25(2):505–12. doi: 10.1021/bi00350a035. - DOI - PubMed
-
- Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266(14):8957–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

