Granulocyte colony-stimulating factor therapy for stem cell mobilization following anterior wall myocardial infarction: the CAPITAL STEM MI randomized trial
- PMID: 24934893
- PMCID: PMC4119168
- DOI: 10.1503/cmaj.140133
Granulocyte colony-stimulating factor therapy for stem cell mobilization following anterior wall myocardial infarction: the CAPITAL STEM MI randomized trial
Abstract
Background: Small studies have yielded divergent results for administration of granulocyte colony-stimulating factor (G-CSF) after acute myocardial infarction. Adequately powered studies involving patients with at least moderate left ventricular dysfunction are lacking.
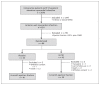
Methods: Patients with left ventricular ejection fraction less than 45% after anterior-wall myocardial infarction were treated with G-CSF (10 μg/kg daily for 4 days) or placebo. After initial randomization of 86 patients, 41 in the placebo group and 39 in the G-CSF group completed 6-month follow-up and underwent measurement of left ventricular ejection fraction by radionuclide angiography.
Results: Baseline and 6-week mean ejection fraction was similar for the G-CSF and placebo groups: 34.8% (95% confidence interval [CI] 32.6%-37.0%) v. 36.4% (95% CI 33.5%-39.2%) at baseline and 39.8% (95% CI 36.2%-43.4%) v. 43.1% (95% CI 39.2%-47.0%) at 6 weeks. However, G-CSF therapy was associated with a lower ejection fraction at 6 months relative to placebo (40.8% [95% CI 37.4%-44.2%] v. 46.0% [95% CI 42.7%-44.3%]). Both groups had improved left ventricular function, but change in left ventricular ejection fraction was lower in patients treated with G-CSF than in those who received placebo (5.7 [95% CI 3.4-8.1] percentage points v. 9.2 [95% CI 6.3-12.1] percentage points). One or more of a composite of several major adverse cardiac events occurred in 8 patients (19%) within each group, with similar rates of target-vessel revascularization.
Interpretation: In patients with moderate left ventricular dysfunction following anterior-wall infarction, G-CSF therapy was associated with a lower 6-month left ventricular ejection fraction but no increased risk of major adverse cardiac events. Future studies of G-CSF in patients with left ventricular dysfunction should be monitored closely for safety.
Trial registration: ClinicalTrials.gov, no. NCT00394498.
© 2014 Canadian Medical Association or its licensors.
Figures
References
-
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–425 - PubMed
-
- Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006;355:1222–32 - PubMed
-
- Schächinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–21 - PubMed
-
- Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 1991;325:164–70 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical