Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm
- PMID: 24934962
- PMCID: PMC4557205
- DOI: 10.1176/appi.ajp.2014.13050595
Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm
Abstract
Objective: The authors assessed the efficacy and safety of combination treatment with varenicline and sustained-release bupropion for smokers who, based on an assessment of initial smoking reduction prior to the quit date, were deemed unlikely to achieve abstinence using nicotine patch treatment.
Method: In a randomized, double-blind, parallel-group adaptive treatment trial, the authors identified 222 cigarette smokers who failed to show a reduction of more than 50% in smoking after 1 week of nicotine patch treatment. Smokers were randomly assigned to receive 12 weeks of varenicline plus bupropion or varenicline plus placebo. The primary outcome measure was continuous smoking abstinence at weeks 8-11 after the target quit date.
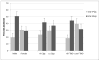
Results: Both treatments were well tolerated. Participants who received the combination treatment had a significantly higher abstinence rate than those who received varenicline plus placebo (39.8% compared with 25.9%; odds ratio=1.89; 95% CI=1.07, 3.35). Combination treatment had a significantly greater effect on abstinence rate in male smokers (odds ratio=4.26; 95% CI=1.73, 10.49) than in female smokers (odds ratio=0.94; 95% CI=0.43, 2.05). It also had a significantly greater effect in highly nicotine-dependent smokers (odds ratio=3.51, 95% CI=1.64, 7.51) than in smokers with lower levels of dependence (odds ratio=0.71, 95% CI=0.28, 1.80).
Conclusions: Among smokers who did not show a sufficient initial response to prequit nicotine patch treatment, combination treatment with varenicline and bupropion proved more efficacious than varenicline alone for male smokers and for smokers with a high degree of nicotine dependence.
Trial registration: ClinicalTrials.gov NCT01303861.
Figures
Comment in
-
Smoking cessation in men and women.Am J Psychiatry. 2014 Nov 1;171(11):1148-50. doi: 10.1176/appi.ajp.2014.14081032. Am J Psychiatry. 2014. PMID: 25756629 No abstract available.
-
Sex difference in response to varenicline for smoking cessation.Am J Psychiatry. 2015 Apr;172(4):394-5. doi: 10.1176/appi.ajp.2015.14111429. Am J Psychiatry. 2015. PMID: 25827037 No abstract available.
-
Response to Gorelick.Am J Psychiatry. 2015 Apr;172(4):395. doi: 10.1176/appi.ajp.2015.14111429r. Am J Psychiatry. 2015. PMID: 25827038 No abstract available.
References
-
- Lando HA, Wilson K. Combating the global tobacco epidemic. Preventive medicine. 2010 Jan-Feb;50(1–2):11–2. - PubMed
-
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. The New England journal of medicine. 2013 Jan 24;368(4):341–50. - PubMed
-
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006 Jul 5;296(1):47–55. - PubMed
-
- Lindson N, Aveyard P. Response to Rose (2011): nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology. 2011 Nov;218(2):459–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical