CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG
- PMID: 24935259
- PMCID: PMC4076586
- DOI: 10.1084/jem.20122192
CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG
Abstract
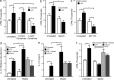
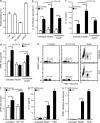
Myelin oligodendrocyte glycoprotein (MOG), a constituent of central nervous system myelin, is an important autoantigen in the neuroinflammatory disease multiple sclerosis (MS). However, its function remains unknown. Here, we show that, in healthy human myelin, MOG is decorated with fucosylated N-glycans that support recognition by the C-type lectin receptor (CLR) DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) on microglia and DCs. The interaction of MOG with DC-SIGN in the context of simultaneous TLR4 activation resulted in enhanced IL-10 secretion and decreased T cell proliferation in a DC-SIGN-, glycosylation-, and Raf1-dependent manner. Exposure of oligodendrocytes to proinflammatory factors resulted in the down-regulation of fucosyltransferase expression, reflected by altered glycosylation at the MS lesion site. Indeed, removal of fucose on myelin reduced DC-SIGN-dependent homeostatic control, and resulted in inflammasome activation, increased T cell proliferation, and differentiation toward a Th17-prone phenotype. These data demonstrate a new role for myelin glycosylation in the control of immune homeostasis in the healthy human brain through the MOG-DC-SIGN homeostatic regulatory axis, which is comprised by inflammatory insults that affect glycosylation. This phenomenon should be considered as a basis to restore immune tolerance in MS.
© 2014 García-Vallejo et al.
Figures
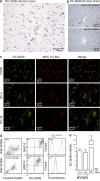
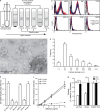

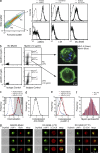

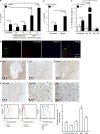
References
-
- Appelmelk B.J., van Die I., van Vliet S.J., Vandenbroucke-Grauls C.M.J.E., Geijtenbeek T.B.H., van Kooyk Y. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635–1639 10.4049/jimmunol.170.4.1635 - DOI - PubMed
-
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragán L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 29:986–997 10.1016/j.immuni.2008.10.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

