Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells
- PMID: 24935351
- PMCID: PMC4114067
- DOI: 10.1042/BSR20140031
Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells
Abstract
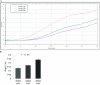
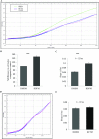
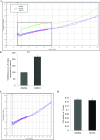
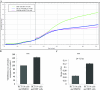
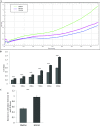
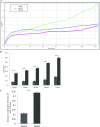
Increasing our knowledge of the mechanisms regulating cell proliferation, migration and invasion are central to understanding tumour progression and metastasis. The local tumour microenvironment contributes to the transformed phenotype in cancer by providing specific environmental cues that alter the cells behaviour and promotes metastasis. Fibroblasts have a strong association with cancer and in recent times there has been some emphasis in designing novel therapeutic strategies that alter fibroblast behaviour in the tumour microenvironment. Fibroblasts produce growth factors, chemokines and many of the proteins laid down in the ECM (extracellular matrix) that promote angiogenesis, inflammation and tumour progression. In this study, we use a label-free RTCA (real-time cell analysis) platform (xCELLigence) to investigate how media derived from human fibroblasts alters cancer cell behaviour. We used a series of complimentary and novel experimental approaches to show HCT116 cells adhere, proliferate and migrate significantly faster in the presence of media from human fibroblasts. As well as this, we used the xCELLigence CIM-plates system to show that HCT116 cells invade matrigel layers aggressively when migrating towards media derived from human fibroblasts. These data strongly suggest that fibroblasts have the ability to increase the migratory and invasive properties of HCT116 cells. This is the first study that provides real-time data on fibroblast-mediated migration and invasion kinetics of colon cancer cells.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

