Bifidobacterium breve MCC-117 Induces Tolerance in Porcine Intestinal Epithelial Cells: Study of the Mechanisms Involved in the Immunoregulatory Effect
- PMID: 24936377
- PMCID: PMC4034327
- DOI: 10.12938/bmfh.33.1
Bifidobacterium breve MCC-117 Induces Tolerance in Porcine Intestinal Epithelial Cells: Study of the Mechanisms Involved in the Immunoregulatory Effect
Abstract
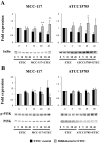
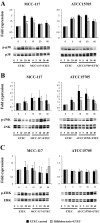
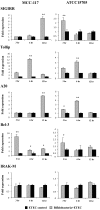
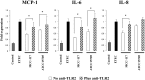
Bifidobacterium breve MCC-117 is able to significantly reduce the expression of inflammatory cytokines in porcine intestinal epithelial (PIE) cells and to improve IL-10 levels in CD4(+)CD25(high) Foxp3(+) lymphocytes in response to heat-stable enterotoxigenic Escherichia coli (ETEC) pathogen-associated molecular patterns (PAMPs), while the immunoregulatory effect of B. adolescentis ATCC15705 was significantly lower than that observed for the MCC-117 strain. Considering the different capacities of the two bifidobacterium strains to activate toll-like receptor (TLR)-2 and their differential immunoregulatory activities in PIE and immune cells, we hypothesized that comparative studies with both strains could provide important information regarding the molecular mechanism(s) involved in the anti-inflammatory activity of bifidobacteria. In this work, we demonstrated that the anti-inflammatory effect of B. breve MCC-117 was achieved by a complex interaction of multiple negative regulators of TLRs as well as inhibition of multiple signaling pathways. We showed that B. breve MCC-117 reduced heat-stable ETEC PAMP-induced NF-κB, p38 MAPK and PI3 K activation and expression of pro-inflammatory cytokines in PIE cells. In addition, we demonstrated that B. breve MCC-117 may activate TLR2 synergistically and cooperatively with one or more other pattern recognition receptors (PRRs), and that interactions may result in a coordinated sum of signals that induce the upregulation of A20, Bcl-3, Tollip and SIGIRR. Upregulation of these negative regulators could have an important physiological impact on maintaining or reestablishing homeostatic TLR signals in PIE cells. Therefore, in the present study, we gained insight into the molecular mechanisms involved in the immunoregulatory effect of B. breve MCC-117.
Keywords: Toll-like receptor 2; Toll-like receptors negative regulators; anti-inflammatory activity; bifidobacteria; porcine intestinal epithelial cells.
Figures

Similar articles
-
Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression.PLoS One. 2013;8(3):e59259. doi: 10.1371/journal.pone.0059259. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555642 Free PMC article.
-
Toll-like receptor-2-activating bifidobacteria strains differentially regulate inflammatory cytokines in the porcine intestinal epithelial cell culture system: finding new anti-inflammatory immunobiotics.FEMS Immunol Med Microbiol. 2011 Oct;63(1):129-39. doi: 10.1111/j.1574-695X.2011.00837.x. Epub 2011 Jul 29. FEMS Immunol Med Microbiol. 2011. PMID: 21711398
-
Advanced application of bovine intestinal epithelial cell line for evaluating regulatory effect of lactobacilli against heat-killed enterotoxigenic Escherichia coli-mediated inflammation.BMC Microbiol. 2013 Mar 7;13:54. doi: 10.1186/1471-2180-13-54. BMC Microbiol. 2013. PMID: 23497067 Free PMC article.
-
Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells.Microorganisms. 2016 Aug 15;4(3):27. doi: 10.3390/microorganisms4030027. Microorganisms. 2016. PMID: 27681921 Free PMC article. Review.
-
Regulation of toll-like receptors-mediated inflammation by immunobiotics in bovine intestinal epitheliocytes: role of signaling pathways and negative regulators.Front Immunol. 2014 Sep 2;5:421. doi: 10.3389/fimmu.2014.00421. eCollection 2014. Front Immunol. 2014. PMID: 25228903 Free PMC article. Review.
Cited by
-
Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs.BMC Immunol. 2014 Jun 19;15:24. doi: 10.1186/1471-2172-15-24. BMC Immunol. 2014. PMID: 24943108 Free PMC article.
-
Effects of Bifidobacterium animalis subsp. lactis IU100 on Immunomodulation and Gut Microbiota in Immunosuppressed Mice.Microorganisms. 2024 Feb 29;12(3):493. doi: 10.3390/microorganisms12030493. Microorganisms. 2024. PMID: 38543544 Free PMC article.
-
Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis.Nutrients. 2020 Jan 15;12(1):219. doi: 10.3390/nu12010219. Nutrients. 2020. PMID: 31952193 Free PMC article.
-
Isolation and Immunocharacterization of Lactobacillus salivarius from the Intestine of Wakame-Fed Pigs to Develop Novel "Immunosynbiotics".Microorganisms. 2019 Jun 6;7(6):167. doi: 10.3390/microorganisms7060167. Microorganisms. 2019. PMID: 31174334 Free PMC article.
-
The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review.Metabolites. 2025 Jul 15;15(7):478. doi: 10.3390/metabo15070478. Metabolites. 2025. PMID: 40710579 Free PMC article. Review.
References
-
- Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171–184 - PubMed
-
- Bron PA, van Baarlen P, Kleerebezem M. 2011. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10: 66–78 - PubMed
-
- Villena J, Aso H, Alvarez S, Kitazawa H. 2012. Porcine toll-like receptors and their crosstalk with immunobiotics: impact in the regulation of gut inflammatory immunity. In: Probiotics: Sources, Types and Health Benefits. NOVA Science publishers, pp. 53–83.
-
- Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. 2006. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J Allergy Clin Immunol 117: 696–702 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
