In vivo axonal transport deficits in a mouse model of fronto-temporal dementia
- PMID: 24936422
- PMCID: PMC4053640
- DOI: 10.1016/j.nicl.2014.02.005
In vivo axonal transport deficits in a mouse model of fronto-temporal dementia
Abstract
Background: Axonal transport is vital for neurons and deficits in this process have been previously reported in a few mouse models of Alzheimer's disease prior to the appearance of plaques and tangles. However, it remains to be determined whether axonal transport is defective prior to the onset of neurodegeneration. The rTg4510 mouse, a fronto-temporal dementia and parkinsonism-17 (FTDP-17) tauopathy model, over-express tau-P301L mutation found in familial forms of FTDP-17, in the forebrain driven by the calcium-calmodulin kinase II promoter. This mouse model exhibits tau pathology, neurodegeneration in the forebrain, and associated behavioral deficits beginning at 4-5 months of age.
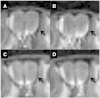
Animal model: rTg4510 transgenic mice were used in these studies. Mice were given 2 μL of MnCl2 in each nostril 1 h prior to Magnetic Resonance Imaging (MRI). Following MnCl2 nasal lavage, mice were imaged using Manganese enhanced Magnetic Resonance Imaging (MEMRI) Protocol with TE = 8.5 ms, TR = 504 ms, FOV = 3.0 cm, matrix size = 128 × 128 × 128, number of cycles = 15 with each cycle taking approximately 2 min, 9 s, and 24 ms using Paravision software (BrukerBioSpin, Billerica, MA). During imaging, body temperature was maintained at 37.0 °C using an animal heating system (SA Instruments, Stony Brook, NY).
Data analysis: Resulting images were analyzed using Paravision software. Regions of interest (ROI) within the olfactory neuronal layer (ONL) and the water phantom consisting of one pixel (ONL) and 9 pixels (water) were selected and copied across each of the 15 cycles. Signal intensities (SI) of ONL and water phantom ROIs were measured. SI values obtained for ONL were then normalized the water phantom SI values. The correlation between normalized signal intensity in the ONL and time were assessed using Prism (GraphPad Software, San Diego, CA).
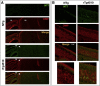
Results: Using the MEMRI technique on 1.5, 3, 5, and 10-month old rTg4510 mice and littermate controls, we found significant axonal transport deficits present in the rTg4510 mice beginning at 3 months of age in an age-dependent manner. Using linear regression analysis, we measured rates of axonal transport at 1.5, 3, 5, and 10 months of age in rTg4510 and WT mice. Axonal transport rates were observed in rTg4510 mice at 48% of WT levels at 3 months, 40% of WT levels at 5 months, and 30% of WT levels at 10 months of age. In order to determine the point at which tau appears in the cortex, we probed for phosphorylated tau levels, and found that pSer262 is present at 3 months of age, not earlier at 1.5 months of age, but observed no pathological tau species until 6 months of age, months after the onset of the transport deficits. In addition, we saw localization of tau in the ONL at 6 months of age.
Discussion: In our study, we identified the presence of age-dependent axonal transport deficits beginning at 3 months of age in rTg4510 mice. We correlated these deficits at 3 months to the presence of hyperphosphorylated tau in the brain and the presence within the olfactory epithelium. We observed tau pathology not only in the soma of these neurons but also within the axons and processes of these neurons. Our characterization of axonal transport in this tauopathy model provides a functional time point that can be used for future therapeutic interventions.
Keywords: Alzheimer's disease; Axonal transport; Fronto-temporal dementia; MEMRI; MRI; Tau.
Figures




Similar articles
-
Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model.Brain Res. 2017 Jun 15;1665:22-35. doi: 10.1016/j.brainres.2017.04.002. Epub 2017 Apr 11. Brain Res. 2017. PMID: 28411086
-
Interaction between a MAPT variant causing frontotemporal dementia and mutant APP affects axonal transport.Neurobiol Aging. 2018 Aug;68:68-75. doi: 10.1016/j.neurobiolaging.2018.03.033. Epub 2018 Apr 5. Neurobiol Aging. 2018. PMID: 29729423 Free PMC article.
-
Progressive Pathological Changes in Neurochemical Profile of the Hippocampus and Early Changes in the Olfactory Bulbs of Tau Transgenic Mice (rTg4510).Neurochem Res. 2017 Jun;42(6):1649-1660. doi: 10.1007/s11064-017-2298-5. Epub 2017 May 18. Neurochem Res. 2017. PMID: 28523532 Free PMC article.
-
Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review.
-
The effects of mild closed head injuries on tauopathy and cognitive deficits in rodents: Primary results in wild type and rTg4510 mice, and a systematic review.Exp Neurol. 2020 Apr;326:113180. doi: 10.1016/j.expneurol.2020.113180. Epub 2020 Jan 11. Exp Neurol. 2020. PMID: 31930992 Free PMC article.
Cited by
-
Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation.Front Neurol. 2021 Jan 7;11:595532. doi: 10.3389/fneur.2020.595532. eCollection 2020. Front Neurol. 2021. PMID: 33488497 Free PMC article. Review.
-
Targeting the ensemble of heterogeneous tau oligomers in cells: A novel small molecule screening platform for tauopathies.Alzheimers Dement. 2019 Nov;15(11):1489-1502. doi: 10.1016/j.jalz.2019.06.4954. Epub 2019 Oct 22. Alzheimers Dement. 2019. PMID: 31653529 Free PMC article.
-
Manganese-Enhanced Magnetic Resonance Imaging: Application in Central Nervous System Diseases.Front Neurol. 2020 Feb 25;11:143. doi: 10.3389/fneur.2020.00143. eCollection 2020. Front Neurol. 2020. PMID: 32161572 Free PMC article. Review.
-
Identification of changes in neuronal function as a consequence of aging and tauopathic neurodegeneration using a novel and sensitive magnetic resonance imaging approach.Neurobiol Aging. 2017 Aug;56:78-86. doi: 10.1016/j.neurobiolaging.2017.04.007. Epub 2017 Apr 18. Neurobiol Aging. 2017. PMID: 28500878 Free PMC article.
-
Manganese-Enhanced MRI: Biological Applications in Neuroscience.Front Neurol. 2015 Jul 10;6:161. doi: 10.3389/fneur.2015.00161. eCollection 2015. Front Neurol. 2015. PMID: 26217304 Free PMC article. Review.
References
-
- Avila J., Lucas J.J., Pérez M., Hernández F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004;84:361–384. - PubMed
-
- Barten D.M., Fanara P., Andorfer C., Hoque N., Wong P.Y.A., Husted K.H., Cadelina G.W., DeCarr L.B., Yang L., Liu V., Fessler C., Protassio J., Riff T., Turner H., Janus C.G., Sankaranarayanan S., Polson C., Meredith J.E., Gray G., Hanna A., Olson R.E., Kim S.-H., Vite G.D., Lee F.Y., Albright C.F. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 2012;32:7137–7145. - PMC - PubMed
-
- Berger Z., Roder H., Hanna A., Carlson A., Rangachari V., Yue M., Wszolek Z., Ashe K., Knight J., Dickson D., Andorfer C., Rosenberry T.L., Lewis J., Hutton M., Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 2007;27:3650–3662. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

