Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis
- PMID: 24936585
- PMCID: PMC4602263
- DOI: 10.1136/annrheumdis-2014-205234
Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis
Abstract
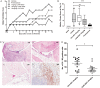
Objective: Previous work has suggested that the granulocyte macrophage colony stimulating factor (GM-CSF)-GM-CSF receptor α axis (GM-CSFRα) may provide a new therapeutic target for the treatment of rheumatoid arthritis (RA). Therefore, we investigated the cellular expression of GM-CSFRα in RA synovial tissue and investigated the effects of anti-GM-CSFRα antibody treatment in vitro and in vivo in a preclinical model of RA.
Methods: We compared GM-CSFRα expression on macrophages positive for CD68 or CD163 on synovial biopsy samples from patients with RA or psoriatic arthritis (PsA) to disease controls. In addition, we studied the effects of CAM-3003, an anti-GM-CSFR antibody in a collagen induced arthritis model of RA in DBA/1 mice. The pharmacokinetic profile of CAM-3003 was studied in naïve CD1(ICR) mice (see online supplement) and used to interpret the results of the pharmacodynamic studies in BALB/c mice.
Results: GM-CSFRα was expressed by CD68 positive and CD163 positive macrophages in the synovium, and there was a significant increase in GM-CSFRα positive cells in patients in patients with RA as well as patients with PsA compared with patients with osteoarthritis and healthy controls. In the collagen induced arthritis model there was a dose dependent reduction of clinical arthritis scores and the number of F4/80 positive macrophages in the inflamed synovium after CAM-3003 treatment. In BALB/c mice CAM-3003 inhibited recombinant GM-CSF mediated margination of peripheral blood monocytes and neutrophils.
Conclusions: The findings support the ongoing development of therapies aimed at interfering with GM-CSF or its receptor in various forms of arthritis, such as RA and PsA.
Keywords: Pharmacokinetics; Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures



References
-
- Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210–21. - PubMed
-
- van Nieuwenhuijze A, Koenders M, Roeleveld D, et al. GM-CSF as a therapeutic target in inflammatory diseases. Mol Immunol 2013;56:675–82. - PubMed
-
- Cornish AL, Campbell IK, McKenzie BS, et al. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:554–9. - PubMed
-
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008;8:533–44. - PubMed
-
- Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors 2004;22:225–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

