Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction
- PMID: 24936744
- PMCID: PMC4222610
- DOI: 10.1097/MPH.0000000000000206
Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction
Abstract
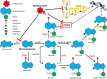
The antileukemic mechanisms of 6-mercaptopurine (6MP) and methotrexate (MTX) maintenance therapy are poorly understood, but the benefits of several years of myelosuppressive maintenance therapy for acute lymphoblastic leukemia are well proven. Currently, there is no international consensus on drug dosing. Because of significant interindividual and intraindividual variations in drug disposition and pharmacodynamics, vigorous dose adjustments are needed to obtain a target degree of myelosuppression. As the normal white blood cell counts vary by patients' ages and ethnicity, and also within age groups, identical white blood cell levels for 2 patients may not reflect the same treatment intensity. Measurements of intracellular levels of cytotoxic metabolites of 6MP and MTX can identify nonadherent patients, but therapeutic target levels remains to be established. A rise in serum aminotransferase levels during maintenance therapy is common and often related to high levels of methylated 6MP metabolites. However, except for episodes of hypoglycemia, serious liver dysfunction is rare, the risk of permanent liver damage is low, and aminotransferase levels usually normalize within a few weeks after discontinuation of therapy. 6MP and MTX dose increments should lead to either leukopenia or a rise in aminotransferases, and if neither is experienced, poor treatment adherence should be considered. The many genetic polymorphisms that determine 6MP and MTX disposition, efficacy, and toxicity have precluded implementation of pharmacogenomics into treatment, the sole exception being dramatic 6MP dose reductions in patients who are homozygous deficient for thiopurine methyltransferase, the enzyme that methylates 6MP and several of its metabolites. In conclusion, maintenance therapy is as important as the more intensive and toxic earlier treatment phases, and often more challenging. Ongoing research address the applicability of drug metabolite measurements for dose adjustments, extensive host genome profiling to understand diversity in treatment efficacy and toxicity, and alternative thiopurine dosing regimens to improve therapy for the individual patient.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Farber S, Diamond LK, Mercer RD, et al. Temporary remissions in acute leukemia in children produced by folic acid antagonist 4-aminopteroylglutamic acid (aminopterin). N Engl J Med. 1948;238:787–793. - PubMed
-
- Burchenal JH, Murphy ML, Ellison RR, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine, in the treatment of leukemia and allied diseases. Blood. 1953;8:965–987. - PubMed
-
- Thomas A. Joe Burchenal and the birth of combination chemotherapy. Br J Haematol. 2006;133:493–503. - PubMed
-
- Frei E, III, Caron M, Levin RH, et al. The effectiveness of combinations of antileukemia agents in inducing and maintaining remission in children with acute leukemia. Blood. 1965;26:642–656. - PubMed
-
- Simone JV. The treatment of acute lymphoblastic leukaemia. Br J Haematol. 1980;45:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

