Cell-type-specific, Aptamer-functionalized Agents for Targeted Disease Therapy
- PMID: 24936916
- PMCID: PMC4078761
- DOI: 10.1038/mtna.2014.21
Cell-type-specific, Aptamer-functionalized Agents for Targeted Disease Therapy
Abstract
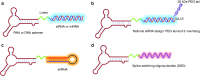
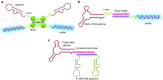
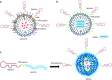
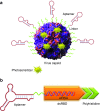
One hundred years ago, Dr. Paul Ehrlich popularized the "magic bullet" concept for cancer therapy in which an ideal therapeutic agent would only kill the specific tumor cells it targeted. Since then, "targeted therapy" that specifically targets the molecular defects responsible for a patient's condition has become a long-standing goal for treating human disease. However, safe and efficient drug delivery during the treatment of cancer and infectious disease remains a major challenge for clinical translation and the development of new therapies. The advent of SELEX technology has inspired many groundbreaking studies that successfully adapted cell-specific aptamers for targeted delivery of active drug substances in both in vitro and in vivo models. By covalently linking or physically functionalizing the cell-specific aptamers with therapeutic agents, such as siRNA, microRNA, chemotherapeutics or toxins, or delivery vehicles, such as organic or inorganic nanocarriers, the targeted cells and tissues can be specifically recognized and the therapeutic compounds internalized, thereby improving the local concentration of the drug and its therapeutic efficacy. Currently, many cell-type-specific aptamers have been developed that can target distinct diseases or tissues in a cell-type-specific manner. In this review, we discuss recent advances in the use of cell-specific aptamers for targeted disease therapy, as well as conjugation strategies and challenges.
Figures



References
-
- Joensuu H. Systemic chemotherapy for cancer: from weapon to treatment. Lancet Oncol. 2008;9:304. - PubMed
-
- Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7:492–508. - PubMed
-
- Shapira S, Lisiansky V, Arber N, Kraus S. Targeted immunotherapy for colorectal cancer: monoclonal antibodies and immunotoxins. Expert Opin Investig Drugs. 2010;19 suppl. 1:S67–S77. - PubMed
-
- Langer R. Drug delivery and targeting. Nature. 1998;392 suppl. 6679:5–10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

