Aminoadamantanes versus other antiviral drugs for chronic hepatitis C
- PMID: 24937404
- PMCID: PMC10542095
- DOI: 10.1002/14651858.CD011132.pub2
Aminoadamantanes versus other antiviral drugs for chronic hepatitis C
Abstract
Background: Hepatitis C virus infection affects around 3% of the world population or approximately 160 million people. A variable proportion (5% to 40%) of the infected people develop clinical symptoms. Hence, hepatitis C virus is a leading cause of liver-related morbidity and mortality with hepatic fibrosis, end-stage liver cirrhosis, and hepatocellular carcinoma as the dominant clinical sequelae. Combination therapy with pegylated (peg) interferon-alpha and ribavirin achieves sustained virological response (that is, undetectable hepatitis C virus RNA in serum by sensitivity testing six months after the end of treatment) in approximately 40% to 80% of treated patients, depending on viral genotype. Recently, a new class of drugs have emerged for hepatitis C infection, the direct acting antivirals, which in combination with standard therapy or alone can lead to sustained virological response in 80% or more of treated patients. Aminoadamantanes, mostly amantadine, are antiviral drugs used for the treatment of patients with chronic hepatitis C. We have previously systematically reviewed amantadine versus placebo or no intervention and found no significant effects of the amantadine on all-cause mortality or liver-related morbidity and on adverse events in patients with hepatitis C. Overall, we did not observe a significant effect of amantadine on sustained virological response. In this review, we systematically review aminoadamantanes versus other antiviral drugs.
Objectives: To assess the beneficial and harmful effects of aminoadamantanes versus other antiviral drugs for patients with chronic hepatitis C virus infection by conducting a systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
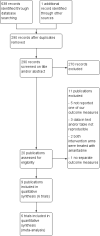
Search methods: The Cochrane Hepato-Biliary Group Controlled Trials Register (1996 to December 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11 of 12, 2013), MEDLINE (1946 to December 2013), EMBASE (1974 to December 2013), Science Citation Index EXPANDED (1900 to December 2013), the WHO International Clinical Trials Registry Platform (www.who.int/ictrp), Google Scholar, and Eudrapharm up to December 2013. Furthermore, full text searches were conducted until December 2013.
Selection criteria: Randomised clinical trials assessing aminoadamantanes in participants with chronic hepatitis C virus infection.
Data collection and analysis: Two authors independently extracted data. RevMan Analysis was used for statistical analysis of dichotomous data using risk ratio (RR) with 95% confidence intervals (CI). Methodological domains were used to assess the risk of systematic errors ('bias'). We used trial sequential analysis to assess risk of random errors ('play of chance').
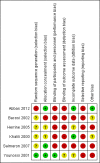
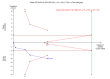
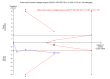
Main results: Six randomised clinical trials with 581 participants with chronic hepatitis C were included. All trials had high risk of bias. The included trials compared amantadine versus other antiviral drugs: ribavirin, mycophenolate mofetil, interferon-alpha, or interferon-gamma. Standard antiviral therapy (interferon-alpha, interferon-alpha plus ribavirin, or peg interferon alpha) was administered equally to the intervention and the control groups in five trials, depending on when the trial was conducted. Four trials compared amantadine versus ribavirin. There were no deaths or liver-related morbidity in the two intervention groups (0/216 (0%) versus 0/211 (0%); 4 trials; very low quality of the evidence). The lower estimated risk for (serious) adverse events leading to treatment discontinuation with amantadine was imprecise (RR 0.56, 95% CI 0.27 to 1.16; based on 10/216 (5%) versus 18/211 (9%) participants in 4 trials; very low quality of the evidence). There were more participants with failure of sustained virological response in the amantadine group than in the ribavirin group (206/216 (96%) versus 176/211 (84%); RR 1.14, 95% CI 1.07 to 1.22, 4 trials; low quality of the evidence). Amantadine versus ribavirin more often failed to achieve end-of follow-up biochemical response (41/46 (89%) versus 31/46 (67%); RR 1.31, 95% CI 1.05 to 1.63; 2 trials; very low quality of the evidence). One trial compared amantadine versus mycophenolate mofetil. There were no significant differences between the two treatment groups, except that amantadine was inferior to mycophenolate mofetil regarding the outcome failure to achieve end-of treatment virological response (low quality of evidence). One trial each compared amantadine versus interferon-alpha or interferon-gamma. Both comparisons showed no significant differences in the treatment outcomes (very low quality of the evidence). The observed effects could be due to real effects, systematic errors (bias), or random errors (play of chance). This possible influence on the observed effect by play of chance is due to the fact that trial sequential analyses could not confirm our findings. We were not able to perform meta-analyses on failure of histological improvement and quality of life due to lack of valid data in all trial comparisons.
Authors' conclusions: This systematic review has identified evidence of very low quality for the key outcomes of all-cause mortality or liver-related morbidity and adverse events in people with chronic hepatitis C when treated with amantadine compared with ribavirin, mycophenolate, interferon-alpha, or interferon-gamma. The timeframe for measuring the composite outcome was insufficient in the included trials. There was low quality evidence that amantadine led to more participants who failed to achieve sustained virological response compared with ribavirin. This observation may be real or caused by systematic errors (bias), but it does not seem to be caused by random error (play of chance). Due to the low quality of the evidence, we are unable to determine definitively whether amantadine is less effective than other antivirals in patients with chronic hepatitis C. As it appears less likely that future trials assessing amantadine or potentially other aminoadamantanes for patients with chronic hepatitis C would show strong benefits, it is probably better to focus on the assessments of other direct acting antiviral drugs. We found no evidence assessing other aminoadamantanes in randomised clinical trials in order to recommend or refute their use.
Conflict of interest statement
Mieke H Lamers: no declarations of interest. Mark Broekman: no declarations of interest. Joost PH Drenth: no declarations of interest. Christian Gluud: no declarations of interest.
Figures
































Update of
- doi: 10.1002/14651858.CD011132
Similar articles
-
Aminoadamantanes for chronic hepatitis C.Cochrane Database Syst Rev. 2014 May 3;2014(5):CD010125. doi: 10.1002/14651858.CD010125.pub2. Cochrane Database Syst Rev. 2014. PMID: 24793264 Free PMC article.
-
Peginterferon plus ribavirin versus interferon plus ribavirin for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Feb 28;2014(2):CD005441. doi: 10.1002/14651858.CD005441.pub3. Cochrane Database Syst Rev. 2014. PMID: 24585509 Free PMC article.
-
Nitazoxanide for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Apr 6;2014(4):CD009182. doi: 10.1002/14651858.CD009182.pub2. Cochrane Database Syst Rev. 2014. PMID: 24706397 Free PMC article.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
References
References to studies included in this review
Abbas 2012 {published data only}
-
- Abbas Z, Raza S, Hamid S, Jafri W. Randomized controlled trial of interferon gamma versus amantadine in combination with interferon alpha and ribavirin for hepatitis C genotype 3 non‐responders and relapsers. Journal of the Pakistan Medical Association 2012;62:338‐43. - PubMed
Bacosi 2002 {published data only}
-
- Bacosi M, Russo F, Dínnocenzo S, Santolamazza M, Miglioresi L, Ursitti A, et al. Amantadine and interferon in the combined treatment of hepatitis C virus in elderly patients. Hepatology Research 2002;22(3):231‐9. - PubMed
Herrine 2005 {published data only}
-
- Herrine SK, Brown RS Jr, Bernstein DE, Ondovik MS, Lentz E, Te H. Peginterferon alpha‐2a combination therapies in chronic hepatitis C patients who relapsed after or had a viral breakthrough on therapy with standard interferon alpha‐2b plus ribavirin: a pilot study of efficacy and safety. Digestive Diseases and Sciences 2005;50(4):719‐26. - PubMed
Khalili 2000 {published data only}
-
- Khalili M, Denham C, Perrillo R. A comparison of combination therapy with interferon and ribavirin vs interferon and amantadine in interferon non‐responders with chronic hepatitis C. Hepatology 1999;28(4):169A.
-
- Khalili M, Denham C, Perrillo R. Interferon and ribavirin versus interferon and amantadine in interferon nonresponders with chronic hepatitis C. American Journal of Gastroenterology 2000;95(5):1284‐9. [PUBMED: 10811340] - PubMed
Salmeron 2007 {published data only}
-
- Salmeron J, Diago M, Andrade R, Perez R, Sola R, Romero M, et al. Induction doses of interferon‐alpha‐2a in combination with ribavirin and/or amantadine for the treatment of chronic hepatitis C in non‐responders to interferon monotherapy: a randomized trial. Journal of Viral Hepatitis 2007;14(2):89‐95. - PubMed
Younossi 2001 {published data only}
-
- Younossi ZM, Mullen KD, Zakko W, Brandt E, Hodnick S, Barnes D, et al. Interferon alpha2B‐ribavirin vs. interferon alpha2B‐amantadine combination in chronic hepatitis C; analyses of a randomized, double‐blind trial. Hepatology 1999;30(4):372A. - PubMed
-
- Younossi ZM, Mullen KD, Zakko W, Brandt E, Hodnick S, Easley K, et al. Interferon alpha2b and ribavirin vs interferon alpha2b and amantadine for chronic hepatitis C (non‐responder): a multi‐center, randomized, double‐blind trial. Hepatology 1998;28(4):284A. - PubMed
-
- Younossi ZM, Mullen KD, Zakko W, Hodnick S, Brand E, Barnes DS, et al. A randomized, double‐blind controlled trial of interferon alpha‐2b and ribavirin vs. interferon alpha‐2b and amantadine for treatment of chronic hepatitis C non‐responder to interferon monotherapy. Journal of Hepatology 2001;34(1):128‐33. [PUBMED: 11211889] - PubMed
References to studies excluded from this review
Bellobuono 2002 {published data only}
-
- Bellobuono A, Poggio P, Jamoletti C, Finazzi R, Covini G, Milazzo L, et al. Combination therapy in naive patients with chronic hepatitis C related to genotype 1: comparison between IFN + ribavirin and IFN + amantadine and between amantadine and ribavirin addition after 3 months in still viremic patients. Hepatology 2002;36:573A.
-
- Bellobuono A, Finazzi R, Covini G, Milazzo L, Magni C, Milella AM, et al. Comparison between IFNalpha 2a + ribavirin and IFNalpha 2a + amantadine combination therapy in naive patients with chronic hepatitis C‐genotype 1, and between amantadine and ribavirin addition in non responders after 3 months. Journal of Hepatology 2002;36(S1):98.
Di Bisceglie 2001 {published data only}
-
- Bisceglie AM, Bernstein DE, Rustgi VR, Gitlin N, Jeffers LJ, Simon D, et al. Pegylated (40 KDA) interferon Alfa‐2A (Pegasys®) in new combination therapies: a report of a randomized, multicenter efficacy and safety study. Journal of Hepatology 2001;34(1):143.
Gerardi 1998 {published data only}
-
- Gerardi R, Berardo C, Marinelli RMA, Delle Monache M, Bacosi M, Ricci GL. Amantadina vs interferon in the treatment of chronic HCV infection. Journal of Hepatology 1998;28(S1):193.
Torre 1999 {published data only}
-
- Torre F, Brizzolara R, Giusto R, Grasso A, Sinelli N, Campo N, et al. HCV‐RNA dynamics in the first month of treatment with IFN daily therapy alone versus combinations with amantadine or ribavirin. Journal of Hepatology 1999;30(1):128.
References to studies awaiting assessment
Ideo 2003 {published data only}
-
- Ideo G, Antonucci G, Attili AF, Babudieri S, Chirianni A, Craxi A, et al. Peg‐IFN alfa‐2a (40KD) (Pegasys®) plus amantadine (AMA) or ribavirin (RBV) in naive patients with chronic hepatitis C (CHC). Hepatology 2003;38(4):728A.
-
- Ideo G, Attili A, Chirianni A, Craxi A, Perri G, Picciotto A, et al. Peg‐IFN alfa‐2a (40KD) (Pegasys) plus amantadine (AMA) or ribavirin (RBV) in naive patients with chronic hepatitis C (CHC). Hepatology 2002;36(4):570A.
-
- Ideo G, Attili, A, Ruggiero G, Craxi A, Perri G, Mangia A, et al. Peg‐IFN alfa‐2a (40KD) (Pegasys) plus amantadine (AMA) or ribavirin (RBV) in naive patients with chronic hepatitis C (CHC). Journal of Hepatology 2003;38(S2):146.
Montaser 2003 {published data only}
-
- Montaser MF. Dimethyl dimethoxy biphenyl dicarboxylate and amantadine hydrochloride combination in treatment of chronic hepatitis C: a pilot study in Egypt. Journal of Hepatology 2003;38(S2):158.
Picciotto 1999 {published data only}
-
- Picciotto A, Torre F, Brizzolara R, Campo N, Giusto R, Sinelli N, et al. Chronic hepatitis C. New therapeutic strategies. Minerva Gastroenterologica e Dietologica 1999;45(3):169‐72. [PUBMED: 16498326] - PubMed
Pimstone 1997 {published data only}
-
- Pimstone NR, Yu AS, Akhondi MM, Lumbra LA. End‐of‐treatment virologic response of chronic hepatitis C with either amantadine or rimantadine. Hepatology 1997;26(4):217A.
Additional references
Altman 2003
Alves Galvao 2012
Asselah 2010
Awad 2010
-
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51(4):1176‐84. - PubMed
Bacon 2011
Brillanti 1999
-
- Brillanti S, Foli M, Tomaso M, Gramantieri L, Masci C, Bolondi L. Pilot study of triple antiviral therapy for chronic hepatitis C in interferon alpha non‐responders. Italian Journal of Gastroenterology and Hepatology 1999;31(2):130‐4. [PUBMED: 10363198] - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
Brok 2010
Butt 2009
-
- Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology 2009;50(2):387‐92. [PUBMED: 19591128] - PubMed
Chen 2012
-
- Chen J, Shi J, Xie WF, Zeng X, Lin Y. Meta‐analysis: amantadine may lower the efficacy of pegylated interferon plus ribavirin in treatment‐naive hepatitis C genotype 1 patients. International Journal of Infectious Diseases 2012;16(10):e748‐e752. - PubMed
Choo 1989
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989;244(4902):359‐62. - PubMed
Chutaputti 2000
-
- Chutaputti A. Adverse effects and other safety aspects of the hepatitis C antivirals. Journal of Gastroenterology and Hepatology 2000;15(Suppl):E156‐E163. - PubMed
Crosby 2009
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 19 March 2014).
Davis 1989
-
- Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC Jr, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1989;321(22):1501‐6. - PubMed
Davis 1998
-
- Davis GL, Esteban‐Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. Interferon alfa‐2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1493‐9. - PubMed
De Clercq 2001
-
- Clercq E. Antiviral drugs: current state of the art. Journal of Clinical Virology 2001;22(1):73‐89. - PubMed
Deltenre 2004
-
- Deltenre P, Henrion J, Canva V, Dharancy S, Texier F, Louvet A, et al. Evaluation of amantadine in chronic hepatitis C: a meta‐analysis. Journal of Hepatology 2004;41(3):462‐73. [PUBMED: 15336450] - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Di Bisceglie 2011
EASL 2014
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of Hepatology 2014;60(2):392‐420. - PubMed
Egger 1997
Egger 2003
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. - PubMed
El‐Serag 2003
-
- El‐Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Annals of Internal Medicine 2003;139(10):817‐23. - PubMed
Ghany 2009
Gluud 1998
-
- Gluud C, Nikolova D. Quality assessment of reports on clinical trials in the Journal of Hepatology. Journal of Hepatology 1998;29(2):321‐7. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [PUBMED: 17316871] - PubMed
Gluud 2014
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 1. Art. No.: LIVER.
Gurusamy 2013
-
- Gurusamy KS, Wilson E, Koretz RL, Allen VB, Davidson BR, Burroughs AK, et al. Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response of relapse to previous antiviral intervention?. PLoS ONE 2013;8(12):e83313. - PMC - PubMed
Guyatt 2008
Hauser 2014
Hauser 2014a
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
Hopewell 2007
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania, USA: Barnett International/PAREXEL, 1997.
Jacobson 2011
-
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. [PUBMED: 21696307] - PubMed
Jefferson 2012
Kenny‐Walsh 1999
-
- Kenny‐Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. New England Journal of Medicine 1999;340(16):1228‐33. - PubMed
Keus 2010
Kim 2009
Kjaergard 1999
-
- Kjaergard LL, Nikolova D, Gluud C. Randomized clinical trials in Hepatology: predictors of quality. Hepatology 1999;30(5):1134‐8. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. - PubMed
Koff 1980
Koretz 1993
-
- Koretz RL, Abbey H, Coleman E, Gitnick G. Non‐A, non‐B post‐transfusion hepatitis. Lookin back in the second decade. Annals of Internal Medicine 1993;119(2):110‐5. - PubMed
Koretz 2013
Lamers 2014
Lauer 2001
-
- Lauer GM, Walker BD. Hepatitis C virus infection. New England Journal of Medicine 2001;345(1):41‐52. - PubMed
Lavanchy 2011
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection 2011;17(2):107‐15. - PubMed
Lawitz 2013
-
- Lawitz E, Mangia A, Wyles D, Rodriguez‐Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New England Journal of Medicine 2013;368(20):1878‐87. [PUBMED: 23607594] - PubMed
Lawitz 2014
-
- Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed‐dose combination with and without ribavirin in treatment‐naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open‐label, randomised, phase 2 trial. Lancet 2014;383(9916):515‐23. - PubMed
Lim 2005
Lundh 2012
McHutchison 1998
-
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1485‐92. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Myers 2002
Needleman 1999
-
- Needleman I. CONSORT. Consolidated Standards of Reporting Trials. British Dental Journal 1999;186(5):207. - PubMed
Poordad 2011
Poynard 1998
-
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352(9138):1426‐32. - PubMed
Reichard 1991
-
- Reichard O, Andersson J, Schvarcz R, Weiland O. Ribavirin treatment for chronic hepatitis C. Lancet 1991;337(8749):1058‐61. - PubMed
Reichard 1993
-
- Reichard O, Yun ZB, Sonnerborg A, Weiland O. Hepatitis C viral RNA titers in serum prior to, during, and after oral treatment with ribavirin for chronic hepatitis C. Journal of Medical Virology 1993;41(2):99‐102. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rodger 2000
-
- Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long‐term outcomes of community‐acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 2000;32(3):582‐7. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012
-
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment (Winchester, England) 2012;16(35):1‐82. [PUBMED: 22989478] - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] - PubMed
Schultz 1995
-
- Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schulz 2012
-
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Internal Journal of Surgery 2011;9(8):672‐7. - PubMed
Seeff 2009
Sherman 2011
Simin 2007
-
- Simin M, Brok J, Stimac D, Gluud C, Gluud LL. Cochrane systematic review: pegylated interferon plus ribavirin vs. interferon plus ribavirin for chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2007;25(10):1153‐62. - PubMed
Simmonds 2005
-
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005;42(4):962‐73. - PubMed
Smith 2004
Smith 2004a
-
- Smith JP, Riley TR 3rd, Bingaman S, Mauger DT. Amantadine therapy for chronic hepatitis C: a dose escalation study. American Journal of Gastroenterology 2004;99(6):1099‐104. - PubMed
Soza 2002
-
- Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology 2002;36(5):1273‐9. - PubMed
Sulkowski 2004
-
- Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha‐2b plus ribavirin combination therapy for chronic hepatitis C virus infection. Journal of Viral Hepatitis 2004;11(3):243‐50. - PubMed
Sulkowski 2014
-
- Sulkowski MS, Gardiner DF, Rodriguez‐Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. New England Journal of Medicine 2014;370(3):211‐21. - PubMed
Sy 2006
Thein 2008
-
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage‐specific fibrosis progression rates in chronic hepatitis C virus infection: a meta‐analysis and meta‐regression. Hepatology 2008;48(2):418‐31. - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 20 March 2014).
Tine 1991
-
- Tine F, Magrin S, Craxi A, Pagliaro L. Interferon for non‐A, non‐B chronic hepatitis. A meta‐analysis of randomised clinical trials. Journal of Hepatology 1991;13(2):192‐9. - PubMed
Ueno 2009
-
- Ueno Y, Sollano JD, Farrell GC. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. Journal of Gastroenterology and Hepatology 2009;24(4):531‐6. - PubMed
Uto 2009
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wiese 2000
-
- Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single‐source outbreak in Germany: a 20‐year multicenter study. Hepatology 2000;32(1):91‐6. - PubMed
Wood 2008
Zeuzem 2011
-
- Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. New England Journal of Medicine 2011;364(25):2417‐28. [PUBMED: 21696308] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

