Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment?
- PMID: 24937545
- PMCID: PMC4263268
- DOI: 10.1148/radiol.14140047
Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment?
Abstract
Purpose: To comprehensively assess brain iron levels in typically developing control subjects and patients with attention deficit hyperactivity disorder (ADHD) when psychostimulant medication history is accounted for.
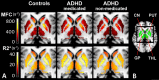
Materials and methods: This prospective study was approved by the institutional review board, and informed consent was obtained. Brain iron was indexed noninvasively by using magnetic resonance (MR) imaging relaxation rates (R2, R2*, R2') and magnetic field correlation (MFC) in the globus pallidus, putamen, caudate nucleus, and thalamus for 22 patients with ADHD (12 medication-naïve patients and 10 with a history of psychostimulant treatment) and 27 control subjects (age range, 8-18 years). Serum iron measures were also collected. Subgroup differences were analyzed with data-appropriate omnibus tests followed by post hoc pairwise comparisons; false discovery rate correction was conducted to control for multiple comparisons.
Results: Medication-naïve ADHD patients had significantly lower striatal and thalamic MFC indexes of brain iron than did control subjects (putamen, P = .012; caudate nucleus, P = .008; thalamus, P = .012) and psychostimulant-medicated ADHD patients (putamen, P = .006; caudate nucleus, P = .010; thalamus, P = .021). Conversely, the MFC indexes in medicated patients were comparable to those in control subjects. No significant differences were detected with R2, R2*, R2', or serum measures.
Conclusion: Lower MFC indexes of striatal and thalamic brain iron in medication-naïve ADHD patients and lack of differences in psychostimulant-medicated patients suggest that MFC indexes of brain iron may represent a noninvasive diagnostic biomarker that responds to psychostimulant treatment.
Figures

References
-
- Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57(11):1410–1415. - PubMed
-
- Swanson JM, Kinsbourne M, Nigg J, et al. . Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 2007;17(1):39–59. - PubMed
-
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry 2012;169(3):264–272. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

