The HYPERFlax trial for determining the anti-HYPERtensive effects of dietary flaxseed in newly diagnosed stage 1 hypertensive patients: study protocol for a randomized, double-blinded, controlled clinical trial
- PMID: 24938224
- PMCID: PMC4073186
- DOI: 10.1186/1745-6215-15-232
The HYPERFlax trial for determining the anti-HYPERtensive effects of dietary flaxseed in newly diagnosed stage 1 hypertensive patients: study protocol for a randomized, double-blinded, controlled clinical trial
Abstract
Background: In 2013 the World Health Organization deemed hypertension as a global crisis as it is the leading risk factor attributed to global mortality. Therefore, there is a great need for effective alternative treatment strategies to combat a condition that affects 40% of adults worldwide. Recently, the FlaxPAD Trial observed a significant reduction in systolic and diastolic blood pressure in hypertensive patients with peripheral arterial disease that consumed 30 g of milled flaxseed per day for one year. However, these patients were already on anti-hypertensive medication. Therefore, there is a need to assess if dietary flaxseed can effectively reduce blood pressure in the absence of peripheral arterial disease and anti-hypertensive medication in newly diagnosed hypertensive patients.
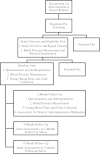
Methods/design: The HYPERFlax Trial is a parallel, superiority, phase II/III, randomized, double-blinded, controlled clinical trial. St. Boniface Hospital and the Health Sciences Centre of Winnipeg, Canada, will recruit 100 participants newly diagnosed with stage 1 hypertension who have yet to be administered anti-hypertensive medication. Participants will be randomly allocated with a 1:1 ratio into a flaxseed or control group and provided food products to consume daily for six months. At baseline, two, four, and six months, participant assessments will include the primary outcome measure, averaged automated blood pressure, and secondary measures: 24-hour food recall, international physical activity questionnaire, anthropometrics, and blood and urine sampling for biochemical analysis. Plasma will be assessed for lipids, metabolomics profiling, and molecules that regulate vascular tone. Urine will be collected for metabolomics profiling. With an estimated dropout rate of 20%, the trial will have a power of 0.80 to detect differences between groups and across time, out of an effect size of 0.7 (SD) at an α level of 0.05.
Discussion: This trial will determine if dietary flaxseed is efficacious over six months as an anti-hypertensive therapy in subjects newly diagnosed with hypertension. If flaxseed can effectively reduce blood pressure as a monotherapy, then flaxseed will provide individuals on a global basis with a cost-effective food-based strategy to control hypertension.
Trial registration: NCT01952340, Registered 24 September 2013.
References
-
- World Health Organization. A Global Brief on Hypertension: Silent killer, Global Public Health Crisis, WHO/DCO/WHD/2013. 2. Geneva, Switzerland: World Health Organization; 2013.
-
- Dupasquier CM, Weber AM, Ander BP, Rampersad PP, Steigerwald S, Wigle JT, Mitchell RW, Kroeger EA, Gilchrist JS, Moghadasian MM, Lukas A, Pierce GN. Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H2987–H2996. - PubMed
-
- Dupasquier CM, Dibrov E, Kneesh AL, Cheung PK, Lee KG, Alexander HK, Yeganeh BK, Moghadasian MH, Pierce GN. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am J Physiol Heart Circ Physiol. 2007;293:H2394–H2402. - PubMed
-
- Bassett CM, McCullough RS, Edel AL, Patenaude A, LaVallee RK, Pierce GN. The alpha-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am J Physiol Heart Circ Physiol. 2011;301:H2220–H2226. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical