Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury
- PMID: 24938728
- PMCID: PMC4229875
- DOI: 10.1186/2051-5960-2-67
Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury
Abstract
Background: Blast-related traumatic brain injury (TBI) is a common cause of injury in the military operations in Iraq and Afghanistan. How the primary blast wave affects the brain is not well understood. The aim of the present study was to examine whether blast exposure affects the cerebral vasculature in a rodent model. We analyzed the brains of rats exposed to single or multiple (three) 74.5 kPa blast exposures, conditions that mimic a mild TBI. Rats were sacrificed 24 hours or between 6 and 10 months after exposure. Blast-induced cerebral vascular pathology was examined by a combination of light microscopy, immunohistochemistry, and electron microscopy.
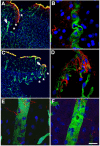
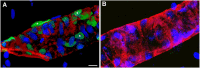
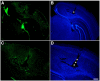
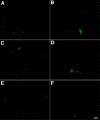
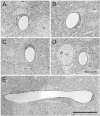
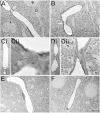
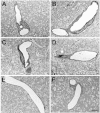
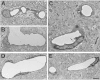
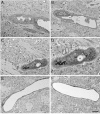
Results: We describe a selective vascular pathology that is present acutely at 24 hours after injury. The vascular pathology is found at the margins of focal shear-related injuries that, as we previously showed, typically follow the patterns of penetrating cortical vessels. However, changes in the microvasculature extend beyond the margins of such lesions. Electron microscopy revealed that microvascular pathology is found in regions of the brain with an otherwise normal neuropil. This initial injury leads to chronic changes in the microvasculature that are still evident many months after the initial blast exposure.
Conclusions: These studies suggest that vascular pathology may be a central mechanism in the induction of chronic blast-related injury.
Figures








References
-
- Bochicchio GV, Lumpkins K, O'Connor J, Simard M, Schaub S, Conway A, Bochicchio K, Scalea TM. Blast injury in a civilian trauma setting is associated with a delay in diagnosis of traumatic brain injury. Am Surg. 2008;74(3):267–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

