A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling
- PMID: 24939893
- PMCID: PMC4345117
- DOI: 10.1126/scisignal.2004838
A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling
Abstract
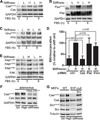
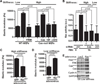
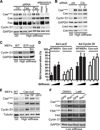
Tissue and extracellular matrix (ECM) stiffness is transduced into intracellular stiffness, signaling, and changes in cellular behavior. Integrins and several of their associated focal adhesion proteins have been implicated in sensing ECM stiffness. We investigated how an initial sensing event is translated into intracellular stiffness and a biologically interpretable signal. We found that a pathway consisting of focal adhesion kinase (FAK), the adaptor protein p130Cas (Cas), and the guanosine triphosphatase Rac selectively transduced ECM stiffness into stable intracellular stiffness, increased the abundance of the cell cycle protein cyclin D1, and promoted S-phase entry. Rac-dependent intracellular stiffening involved its binding partner lamellipodin, a protein that transmits Rac signals to the cytoskeleton during cell migration. Our findings establish that mechanotransduction by a FAK-Cas-Rac-lamellipodin signaling module converts the external information encoded by ECM stiffness into stable intracellular stiffness and mechanosensitive cell cycling. Thus, lamellipodin is important not only in controlling cellular migration but also for regulating the cell cycle in response to mechanical signals.
Copyright © 2014, American Association for the Advancement of Science.
Figures



References
-
- Paszek M, Weaver V. The Tension Mounts: Mechanics Meets Morphogenesis and Malignancy. Journal of Mammary Gland Biology and Neoplasia. 2004;9:325–342. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. - PubMed
-
- Kothapalli D, Liu S-L, Bae Yong H, Monslow J, Xu T, Hawthorne Elizabeth A, Byfield Fitzroy J, Castagnino P, Rao S, Rader Daniel J, Pure E, Phillips Michael C, Lund-Katz S, Janmey Paul A, Assoian Richard K. Cardiovascular Protection by ApoE and ApoE-HDL Linked to Suppression of ECM Gene Expression and Arterial Stiffening. Cell Reports. 2012;2:1259–1271. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

