N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-di-hydro-1H-pyrazol-4-yl)-2-(4-nitro-phen-yl)acetamide
- PMID: 24940224
- PMCID: PMC4051092
- DOI: 10.1107/S1600536814009738
N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-di-hydro-1H-pyrazol-4-yl)-2-(4-nitro-phen-yl)acetamide
Abstract
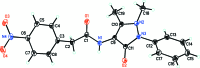
In the title compound, C19H18N4O4, the nitro-phenyl and phenyl rings are twisted by 67.0 (6) and 37.4 (4)°, respectively, with respect to the pyrazole ring plane [maximum deviation = 0.0042 (16) Å]. The dihedral angle between the mean planes of the phenyl rings is 59.3 (3)°. The amide group, with a C-N-C-C torsion angle of 177.54 (13)°, is twisted away from the plane of the pyrazole ring in an anti-periplanar conformation. In the crystal, N-H⋯O hydrogen bonds involving the carbonyl group on the pyrazole ring and the amide group, together with weak C-H⋯O inter-actions forming R 2 (2)(10) graph-set motifs, link the mol-ecules into chains along [100]. Additional weak C-H⋯O inter-actions involving the nitro-phenyl rings further link the mol-ecules along [001], also forming R 2 (2)(10) graph-set motifs, thereby generating (010) layers.
Figures

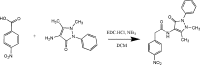
References
-
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Chandrakantha, B., Isloor, A. M., Sridharan, K., Philip, R., Shetty, P. & Padaki, M. (2013). Arab. J Chem. 6, 97–102.
LinkOut - more resources
Full Text Sources
Other Literature Sources
