Pluripotent muse cells derived from human adipose tissue: a new perspective on regenerative medicine and cell therapy
- PMID: 24940477
- PMCID: PMC4041046
- DOI: 10.1186/2001-1326-3-12
Pluripotent muse cells derived from human adipose tissue: a new perspective on regenerative medicine and cell therapy
Abstract
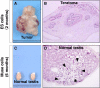
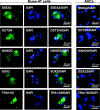
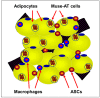
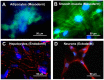
In 2010, Multilineage Differentiating Stress Enduring (Muse) cells were introduced to the scientific community, offering potential resolution to the issue of teratoma formation that plagues both embryonic stem (ES) and induced pluripotent (iPS) stem cells. Isolated from human bone marrow, dermal fibroblasts, adipose tissue and commercially available adipose stem cells (ASCs) under severe cellular stress conditions, Muse cells self-renew in a controlled manner and do not form teratomas when injected into immune-deficient mice. Furthermore, Muse cells express classic pluripotency markers and differentiate into cells from the three embryonic germ layers both spontaneously and under media-specific induction. When transplanted in vivo, Muse cells contribute to tissue generation and repair. This review delves into the aspects of Muse cells that set them apart from ES, iPS, and various reported adult pluripotent stem cell lines, with specific emphasis on Muse cells derived from adipose tissue (Muse-AT), and their potential to revolutionize the field of regenerative medicine and stem cell therapy.
Keywords: Adult pluripotent stem cells; Muse cells; Non-tumorigenic; Quiescence; Regenerative medicine.
Figures
References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;3(5391):1145–1147. - PubMed
-
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;3(1):55–70. doi: 10.1016/j.stem.2007.05.014. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
