Surface Entrapment of Fibronectin on Electrospun PLGA Scaffolds for Periodontal Tissue Engineering
- PMID: 24940563
- PMCID: PMC4048976
- DOI: 10.1089/biores.2014.0015
Surface Entrapment of Fibronectin on Electrospun PLGA Scaffolds for Periodontal Tissue Engineering
Abstract
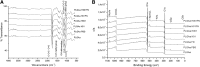
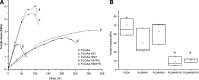
Nowadays, the challenge in the tissue engineering field consists in the development of biomaterials designed to regenerate ad integrum damaged tissues. Despite the current use of bioresorbable polyesters such as poly(l-lactide) (PLA), poly(d,l-lactide-co-glycolide) (PLGA), and poly-ɛ-caprolactone in soft tissue regeneration researches, their hydrophobic properties negatively influence the cell adhesion. Here, to overcome it, we have developed a fibronectin (FN)-functionalized electrospun PLGA scaffold for periodontal ligament regeneration. Functionalization of electrospun PLGA scaffolds was performed by alkaline hydrolysis (0.1 or 0.01 M NaOH). Then, hydrolyzed scaffolds were coated by simple deposition of an FN layer (10 μg/mL). FN coating was evidenced by X-ray photoelectron analysis. A decrease of contact angle and greater cell adhesion to hydrolyzed, FN-coated PLGA scaffolds were noticed. Suitable degradation behavior without pH variations was observed for all samples up to 28 days. All treated materials presented strong shrinkage, fiber orientation loss, and collapsed fibers. However, functionalization process using 0.01 M NaOH concentration resulted in unchanged scaffold porosity, preserved chemical composition, and similar mechanical properties compared with untreated scaffolds. The proposed simplified method to functionalize electrospun PLGA fibers is an efficient route to make polyester scaffolds more biocompatible and shows potential for tissue engineering.
Keywords: biomaterials; proteins; tissue engineering.
Figures




References
-
- Vaquette C, Fan W, Xiao Y, et al. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33:5560–5573 - PubMed
-
- Chang NJ, Lin CC, Li CF, et al. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials. 2012;33:3153–3163 - PubMed
-
- Reis ECC, Borges APB, Araujo MVF, et al. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32:9244–9253 - PubMed
-
- Slots J. Periodontology: past, present, perspectives. Periodontology 2000. 2013;62:7–19 - PubMed
-
- Susin C, Wikesjo UME. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontology 2000. 2013;62:232–242 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
