Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen
- PMID: 24940786
- PMCID: PMC4070071
- DOI: 10.1016/j.bpj.2014.05.014
Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen
Abstract
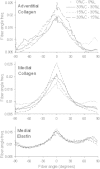
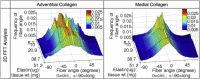
The complex network structure of elastin and collagen extracellular matrix (ECM) forms the primary load bearing components in the arterial wall. The structural and mechanobiological interactions between elastin and collagen are important for properly functioning arteries. Here, we examined the elastin and collagen organization, realignment, and recruitment by coupling mechanical loading and multiphoton imaging. Two-photon excitation fluorescence and second harmonic generation methods were performed with a multiphoton video-rate microscope to capture real time changes to the elastin and collagen structure during biaxial deformation. Enzymatic removal of elastin was performed to assess the structural changes of the remaining collagen structure. Quantitative analysis of the structural changes to elastin and collagen was made using a combination of two-dimensional fast Fourier transform and fractal analysis, which allows for a more complete understanding of structural changes. Our study provides new quantitative evidence, to our knowledge on the sequential engagement of different arterial ECM components in response to mechanical loading. The adventitial collagen exists as large wavy bundles of fibers that exhibit fiber engagement after 20% strain. The medial collagen is engaged throughout the stretching process, and prominent elastic fiber engagement is observed up to 20% strain after which the engagement plateaus. The fiber orientation distribution functions show remarkably different changes in the ECM structure in response to mechanical loading. The medial collagen shows an evident preferred circumferential distribution, however the fiber families of adventitial collagen are obscured by their waviness at no or low mechanical strains. Collagen fibers in both layers exhibit significant realignment in response to unequal biaxial loading. The elastic fibers are much more uniformly distributed and remained relatively unchanged due to loading. Removal of elastin produces similar structural changes in collagen as mechanical loading. Our study suggests that the elastic fibers are under tension and impart an intrinsic compressive stress on the collagen.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Holzapfel G.A. Collagen in arterial walls: biomechanical aspects. In: Fratzl P., editor. Collagen. Structure and Mechanics. Springer-Verlag; Heidelberg: 2008. pp. 285–324.
-
- Kelleher C.M., McLean S.E., Mecham R.P. Vascular extracellular matrix and aortic development. Curr. Top. Dev. Biol. 2004;62:153–188. - PubMed
-
- Burton A.C. Relation of structure to function of the tissues of the wall of blood vessels. Physiol. Rev. 1954;34:619–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

