Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts
- PMID: 24940999
- PMCID: PMC4062422
- DOI: 10.1371/journal.pone.0099348
Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts
Abstract
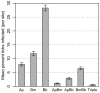
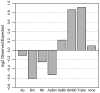
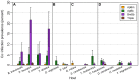
Humans in the northeastern and midwestern United States are at increasing risk of acquiring tickborne diseases--not only Lyme disease, but also two emerging diseases, human granulocytic anaplasmosis and human babesiosis. Co-infection with two or more of these pathogens can increase the severity of health impacts. The risk of co-infection is intensified by the ecology of these three diseases because all three pathogens (Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti) are transmitted by the same vector, blacklegged ticks (Ixodes scapularis), and are carried by many of the same reservoir hosts. The risk of exposure to multiple pathogens from a single tick bite and the sources of co-infected ticks are not well understood. In this study, we quantify the risk of co-infection by measuring infection prevalence in 4,368 questing nymphs throughout an endemic region for all three diseases (Dutchess County, NY) to determine if co-infections occur at frequencies other than predicted by independent assortment of pathogens. Further, we identify sources of co-infection by quantifying rates of co-infection on 3,275 larval ticks fed on known hosts. We find significant deviations of levels of co-infection in questing nymphs, most notably 83% more co-infection with Babesia microti and Borrelia burgdorferi than predicted by chance alone. Further, this pattern of increased co-infection was observed in larval ticks that fed on small mammal hosts, but not on meso-mammal, sciurid, or avian hosts. Co-infections involving A. phagocytophilum were less common, and fewer co-infections of A. phagocytophilum and B. microti than predicted by chance were observed in both questing nymphs and larvae fed on small mammals. Medical practitioners should be aware of the elevated risk of B. microti/B. burgdorferi co-infection.
Conflict of interest statement
Figures
References
-
- Krause P, McKay K, Gadbaw J, Christianson D, Closter L, et al. (2003) Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg 68: 431–436 doi:not available - PubMed
-
- Ostfeld RS (2010) Lyme disease: the ecology of a complex system. New York, NY, USA: Oxford University Press. 232 p.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

