Sharing clinical trial data on patient level: opportunities and challenges
- PMID: 24942505
- PMCID: PMC4314673
- DOI: 10.1002/bimj.201300283
Sharing clinical trial data on patient level: opportunities and challenges
Abstract
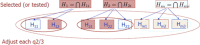
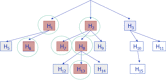
In recent months one of the most controversially discussed topics among regulatory agencies, the pharmaceutical industry, journal editors, and academia has been the sharing of patient-level clinical trial data. Several projects have been started such as the European Medicines Agency´s (EMA) "proactive publication of clinical trial data", the BMJ open data campaign, or the AllTrials initiative. The executive director of the EMA, Dr. Guido Rasi, has recently announced that clinical trial data on patient level will be published from 2014 onwards (although it has since been delayed). The EMA draft policy on proactive access to clinical trial data was published at the end of June 2013 and open for public consultation until the end of September 2013. These initiatives will change the landscape of drug development and publication of medical research. They provide unprecedented opportunities for research and research synthesis, but pose new challenges for regulatory authorities, sponsors, scientific journals, and the public. Besides these general aspects, data sharing also entails intricate biostatistical questions such as problems of multiplicity. An important issue in this respect is the interpretation of multiple statistical analyses, both prospective and retrospective. Expertise in biostatistics is needed to assess the interpretation of such multiple analyses, for example, in the context of regulatory decision-making by optimizing procedural guidance and sophisticated analysis methods.
Keywords: EMA draft policy/0070; Open access to clinical trial data; Raw data; Secondary research; Transparency; Validation.
© 2014 The Author. Biometrical Journal published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Aharoni E, Rosset S. Generalized Alpha Investing: Definitions, Optimality Results, and Application to Public Databases. Journal of the Royal Statistical Society Series B. 2013 doi: 10.1111/rssb.12048. - DOI
-
- Alvarez Y, Hidalgo A, Maignen F, Slattery J. Validation of Statistical Signal Detection Procedures in EudraVigilance Post Authorisation Data: a retrospective evaluation of the potential for earlier signalling. Drug Safety. 2010;33:475–487. - PubMed
-
- Benjamini Y, Bogomolov M. Selective inference on multiple families of hypotheses. Journal of the Royal Statistical Society Series B. 2014;76:297–318.
-
- Buyse M, George SL, Evans S, Geller NL, Ranstam J, Scherrer B, Lesaffre E, Murray G, Edler L, Hutton J, Colton T, Lachenbruch P, Verma BL. The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials. Statistics in Medicine. 1999;18:3435–3451. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

