Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients
- PMID: 24942562
- PMCID: PMC4120239
- DOI: 10.1038/ncomms5167
Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients
Abstract
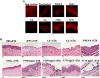
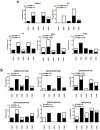
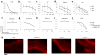
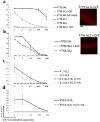
Pemphigus vulgaris (PV) is a potentially fatal blistering disease caused by autoantibodies (autoAbs) against desmoglein 3 (Dsg3). Here, we clone anti-Dsg3 antibodies (Abs) from four PV patients and identify pathogenic VH1-46 autoAbs from all four patients. Unexpectedly, VH1-46 autoAbs had relatively few replacement mutations. We reverted antibody somatic mutations to their germline sequences to determine the requirement of mutations for autoreactivity. Three of five VH1-46 germline-reverted Abs maintain Dsg3 binding, compared with zero of five non-VH1-46 germline-reverted Abs. Site-directed mutagenesis of VH1-46 Abs demonstrates that acidic amino-acid residues introduced by somatic mutation or heavy chain VDJ recombination are necessary and sufficient for Dsg3 binding. Our data suggest that VH1-46 autoantibody gene usage is commonly found in PV because VH1-46 Abs require few to no mutations to acquire Dsg3 autoreactivity, which may favour their early selection. Common VH gene usage indicates common humoral immune responses, even among unrelated patients.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- K08 AR053505/AR/NIAMS NIH HHS/United States
- T15 LM007056/LM/NLM NIH HHS/United States
- AR057001/AR/NIAMS NIH HHS/United States
- HL078726-S1/HL/NHLBI NIH HHS/United States
- R03 AI092379/AI/NIAID NIH HHS/United States
- R03AI092379/AI/NIAID NIH HHS/United States
- T15-LM07056/LM/NLM NIH HHS/United States
- R01 HL078726/HL/NHLBI NIH HHS/United States
- P30-AR057217/AR/NIAMS NIH HHS/United States
- R01 AR057001/AR/NIAMS NIH HHS/United States
- T32 AR007465/AR/NIAMS NIH HHS/United States
- AR053505/AR/NIAMS NIH HHS/United States
- T32-AR007465/AR/NIAMS NIH HHS/United States
- P30 AR057217/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

