Exon skipping restores dystrophin expression, but fails to prevent disease progression in later stage dystrophic dko mice
- PMID: 24942628
- PMCID: PMC4167372
- DOI: 10.1038/gt.2014.53
Exon skipping restores dystrophin expression, but fails to prevent disease progression in later stage dystrophic dko mice
Abstract
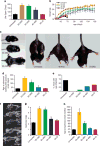
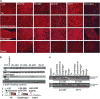
Antisense therapy with both chemistries of phosphorodiamidate morpholino oligomers (PMOs) and 2'-O-methyl phosphorothioate has demonstrated the capability to induce dystrophin expression in Duchenne muscular dystrophy (DMD) patients in phase II-III clinical trials with benefit in muscle functions. However, potential of the therapy for DMD at different stages of the disease progression is not understood. In this study, we examined the effect of peptide-conjugated PMO (PPMO)-mediated exon skipping on disease progression of utrophin-dystrophin-deficient mice (dko) of four age groups (21-29, 30-39, 40-49 and 50+ days), representing diseases from early stage to advanced stage with severe kyphosis. Biweekly intravenous (i.v.) administration of the PPMO restored the dystrophin expression in nearly 100% skeletal muscle fibers in all age groups. This was associated with the restoration of dystrophin-associated proteins including functional glycosylated dystroglycan and neuronal nitric synthase. However, therapeutic outcomes clearly depended on severity of the disease at the time the treatment started. The PPMO treatment alleviated the disease pathology and significantly prolonged the life span of the mice receiving treatment at younger age with mild phenotype. However, restoration of high levels of dystrophin expression failed to prevent disease progression to the mice receiving treatment when disease was already at advanced stage. The results could be critical for design of clinical trials with antisense therapy to DMD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Dunckley MG, Manoharan M, Villiet P, Eperon IC, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

