Intestinal IgA production and its role in host-microbe interaction
- PMID: 24942683
- PMCID: PMC4174397
- DOI: 10.1111/imr.12189
Intestinal IgA production and its role in host-microbe interaction
Abstract
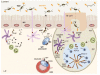
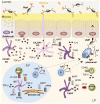
Complex and diverse communities of bacteria establish mutualistic and symbiotic relationships with the gut after birth. The intestinal immune system responds to bacterial colonization by acquiring a state of hypo-responsiveness against commensals and active readiness against pathogens. The resulting homeostatic balance involves a continuous dialog between the microbiota and lymphocytes with the intermediation of epithelial and dendritic cells. This dialog causes massive production of immunoglobulin A (IgA), a non-inflammatory antibody specialized in mucosal protection. Here, we discuss recent advances on the regulation of intestinal IgA responses and their role in host-microbe interaction.
Keywords: B cells; T cells; class switching; dendritic cells; epithelial cells; immunoglobulin; mucosal immunity.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. - PubMed
-
- Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

