Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation
- PMID: 24942756
- PMCID: PMC4082460
- DOI: 10.1158/2326-6066.CIR-14-0027
Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation
Abstract
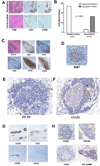
Pancreatic ductal adenocarcinoma (PDAC) is considered a "nonimmunogenic" neoplasm. Single-agent immunotherapies have failed to demonstrate significant clinical activity in PDAC and other "nonimmunogenic" tumors, in part due to a complex tumor microenvironment (TME) that provides a formidable barrier to immune infiltration and function. We designed a neoadjuvant and adjuvant clinical trial comparing an irradiated, granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting, allogeneic PDAC vaccine (GVAX) given as a single agent or in combination with low-dose cyclophosphamide to deplete regulatory T cells (Treg) as a means to study how the TME is altered by immunotherapy. Examination of resected PDACs revealed the formation of vaccine-induced intratumoral tertiary lymphoid aggregates in 33 of 39 patients 2 weeks after vaccine treatment. Immunohistochemical analysis showed these aggregates to be regulatory structures of adaptive immunity. Microarray analysis of microdissected aggregates identified gene-expression signatures in five signaling pathways involved in regulating immune-cell activation and trafficking that were associated with improved postvaccination responses. A suppressed Treg pathway and an enhanced Th17 pathway within these aggregates were associated with improved survival, enhanced postvaccination mesothelin-specific T-cell responses, and increased intratumoral Teff:Treg ratios. This study provides the first example of immune-based therapy converting a "nonimmunogenic" neoplasm into an "immunogenic" neoplasm by inducing infiltration of T cells and development of tertiary lymphoid structures in the TME. Post-GVAX T-cell infiltration and aggregate formation resulted in the upregulation of immunosuppressive regulatory mechanisms, including the PD-1-PD-L1 pathway, suggesting that patients with vaccine-primed PDAC may be better candidates than vaccine-naïve patients for immune checkpoint and other immunomodulatory therapies.
©2014 American Association for Cancer Research.
Conflict of interest statement
The other authors have no conflict to disclose.
Figures






References
-
- Society AC. Cancer Facts & Figures. Atlanta: 2012.
-
- Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Letters. 2009;279:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

