Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study
- PMID: 24942789
- PMCID: PMC4062705
- DOI: 10.1136/bmj.g3596
Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study
Abstract
Objective: To investigate if the widely publicized warnings in 2003 from the US Food and Drug Administration about a possible increased risk of suicidality with antidepressant use in young people were associated with changes in antidepressant use, suicide attempts, and completed suicides among young people.
Design: Quasi-experimental study assessing changes in outcomes after the warnings, controlling for pre-existing trends.
Setting: Automated healthcare claims data (2000-10) derived from the virtual data warehouse of 11 health plans in the US Mental Health Research Network.
Participants: Study cohorts included adolescents (around 1.1 million), young adults (around 1.4 million), and adults (around 5 million).
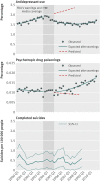
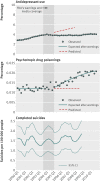
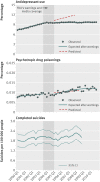
Main outcome measures: Rates of antidepressant dispensings, psychotropic drug poisonings (a validated proxy for suicide attempts), and completed suicides.
Results: Trends in antidepressant use and poisonings changed abruptly after the warnings. In the second year after the warnings, relative changes in antidepressant use were -31.0% (95% confidence interval -33.0% to -29.0%) among adolescents, -24.3% (-25.4% to -23.2%) among young adults, and -14.5% (-16.0% to -12.9%) among adults. These reflected absolute reductions of 696, 1216, and 1621 dispensings per 100,000 people among adolescents, young adults, and adults, respectively. Simultaneously, there were significant, relative increases in psychotropic drug poisonings in adolescents (21.7%, 95% confidence interval 4.9% to 38.5%) and young adults (33.7%, 26.9% to 40.4%) but not among adults (5.2%, -6.5% to 16.9%). These reflected absolute increases of 2 and 4 poisonings per 100,000 people among adolescents and young adults, respectively (approximately 77 additional poisonings in our cohort of 2.5 million young people). Completed suicides did not change for any age group.
Conclusions: Safety warnings about antidepressants and widespread media coverage decreased antidepressant use, and there were simultaneous increases in suicide attempts among young people. It is essential to monitor and reduce possible unintended consequences of FDA warnings and media reporting.
© Lu et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Communicating the harmful effects of medicines.BMJ. 2014 Jun 18;348:g4047. doi: 10.1136/bmj.g4047. BMJ. 2014. PMID: 24942829 No abstract available.
-
Antidepressants increase, rather than decrease, risk of suicidal behaviours in younger patients.BMJ. 2014 Oct 9;349:g5626. doi: 10.1136/bmj.g5626. BMJ. 2014. PMID: 25300283 No abstract available.
-
Study of study of changes in antidepressant use after FDA warnings is not reliable.BMJ. 2014 Oct 9;349:g5623. doi: 10.1136/bmj.g5623. BMJ. 2014. PMID: 25300434 No abstract available.
-
Link between FDA antidepressant warnings and increased suicide attempts in young people is questionable.BMJ. 2014 Oct 9;349:g5614. doi: 10.1136/bmj.g5614. BMJ. 2014. PMID: 25301267 Free PMC article. No abstract available.
-
Study findings on FDA antidepressant warnings and suicide attempts in young people: a false alarm?BMJ. 2014 Oct 9;349:g5645. doi: 10.1136/bmj.g5645. BMJ. 2014. PMID: 25301785 No abstract available.
-
Impossible to draw meaningful conclusions from study of changes in antidepressant use after FDA warnings.BMJ. 2014 Oct 9;349:g5643. doi: 10.1136/bmj.g5643. BMJ. 2014. PMID: 25301791 No abstract available.
-
Proxy for suicide attempts was inappropriate in study of changes in antidepressant use after FDA warnings.BMJ. 2014 Oct 9;349:g5644. doi: 10.1136/bmj.g5644. BMJ. 2014. PMID: 25301794 No abstract available.
-
More data needed to interpret link between suicide and FDA warning on antidepressants.BMJ. 2014 Oct 9;349:g5616. doi: 10.1136/bmj.g5616. BMJ. 2014. PMID: 25301797 No abstract available.
-
Authors' reply to Olfson and Schoenbaum, Nardo, Bartlett, Moore, Case, Gøtzsche, and Barber and colleagues.BMJ. 2014 Oct 9;349:g5722. doi: 10.1136/bmj.g5722. BMJ. 2014. PMID: 25301799 No abstract available.
-
Authors' reply to Mosholder and colleagues.BMJ. 2014 Oct 29;349:g6516. doi: 10.1136/bmj.g6516. BMJ. 2014. PMID: 25354502 No abstract available.
-
Reservations about study on antidepressant use by young people and suicidal behaviour after FDA warnings.BMJ. 2014 Oct 29;349:g6503. doi: 10.1136/bmj.g6503. BMJ. 2014. PMID: 25355684 No abstract available.
References
-
- Kaizar EE, Greenhouse JB, Seltman H, Kelleher K. Do antidepressants cause suicidality in children? A Bayesian meta-analysis. Clin Trials 2006;3:73-90; discussion 91-78. - PubMed
-
- Leckman JF, King RA. A developmental perspective on the controversy surrounding the use of SSRIs to treat pediatric depression. Am J Psychiatry 2007;164:1304-6. - PubMed
-
- Simon GE. The antidepressant quandary—considering suicide risk when treating adolescent depression. N Engl J Med 2006;355:2722-3. - PubMed
-
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-9. - PubMed
-
- Olfson M, Marcus SC. A case-control study of antidepressants and attempted suicide during early phase treatment of major depressive episodes. J Clin Psychiatry 2008;69:425-32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
