Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study
- PMID: 24943065
- PMCID: PMC4165604
- DOI: 10.1016/S2213-8587(14)70114-7
Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study
Abstract
Background: Closed-loop insulin delivery is a promising option to improve glycaemic control and reduce the risk of hypoglycaemia. We aimed to assess whether overnight home use of automated closed-loop insulin delivery would improve glucose control.
Methods: We did this open-label, multicentre, randomised controlled, crossover study between Dec 1, 2012, and Dec 23, 2014, recruiting patients from three centres in the UK. Patients aged 18 years or older with type 1 diabetes were randomly assigned to receive 4 weeks of overnight closed-loop insulin delivery (using a model-predictive control algorithm to direct insulin delivery), then 4 weeks of insulin pump therapy (in which participants used real-time display of continuous glucose monitoring independent of their pumps as control), or vice versa. Allocation to initial treatment group was by computer-generated permuted block randomisation. Each treatment period was separated by a 3-4 week washout period. The primary outcome was time spent in the target glucose range of 3·9-8·0 mmol/L between 0000 h and 0700 h. Analyses were by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01440140.
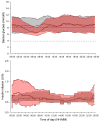
Findings: We randomly assigned 25 participants to initial treatment in either the closed-loop group or the control group, patients were later crossed over into the other group; one patient from the closed-loop group withdrew consent after randomisation, and data for 24 patients were analysed. Closed loop was used over a median of 8·3 h (IQR 6·0-9·6) on 555 (86%) of 644 nights. The proportion of time when overnight glucose was in target range was significantly higher during the closed-loop period compared to during the control period (mean difference between groups 13·5%, 95% CI 7·3-19·7; p=0·0002). We noted no severe hypoglycaemic episodes during the control period compared with two episodes during the closed-loop period; these episodes were not related to closed-loop algorithm instructions.
Interpretation: Unsupervised overnight closed-loop insulin delivery at home is feasible and could improve glucose control in adults with type 1 diabetes.
Funding: Diabetes UK.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
The artificial pancreas: a digital-age treatment for diabetes.Lancet Diabetes Endocrinol. 2014 Sep;2(9):679-81. doi: 10.1016/S2213-8587(14)70126-3. Epub 2014 Jun 16. Lancet Diabetes Endocrinol. 2014. PMID: 24943066 Free PMC article. No abstract available.
References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. - PubMed
-
- Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med. 1991;90(4):450–9. - PubMed
-
- McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19(5):792–9. - PubMed
-
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11(4):372–8. - PubMed
-
- Rankin D, Cooke DD, Clark M, et al. How and why do patients with Type 1 diabetes sustain their use of flexible intensive insulin therapy? A qualitative longitudinal investigation of patients’ self-management practices following attendance at a Dose Adjustment for Normal Eating (DAFNE) course. Diabet Med. 2011;28(5):532–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

