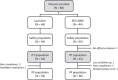
A randomised, double-blind study of polyethylene glycol 4000 and lactulose in the treatment of constipation in children
- PMID: 24943105
- PMCID: PMC4075982
- DOI: 10.1186/1471-2431-14-153
A randomised, double-blind study of polyethylene glycol 4000 and lactulose in the treatment of constipation in children
Abstract
Background: Chronic constipation is frequent in children. The objective of this study is to compare the efficacy and safety of PEG 4000 and lactulose for the treatment of chronic constipation in young children.
Methods: This randomised, double-blind study enrolled 88 young children aged 12 to 36 months, who were randomly assigned to receive lactulose (3.3 g per day) or PEG 4000 (8 g per day) for four weeks. The primary efficacy variable was stool frequency during the fourth week of treatment. Secondary outcomes were the number and frequency of subjective symptoms associated with defecation at each visit.
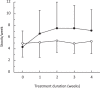
Results: Stool frequency was comparable in the two groups at baseline (lactulose: 0.7 ± 0.5; PEG 4000: 0.5 ± 0.55). Mean stool frequency increased from 0.70 ± 0.50 stools/day at baseline to 0.80 ± 0.41 at Week 4 in the lactulose group and from 0.50 ± 0.55 to 1.10 ± 0.55 stools/day in the PEG 4000 group. A significant difference was observed in the adjusted mean change from baseline, which was 0.15 stools/day in the lactulose group and 0.51 stools/day in the PEG 4000 group, with a least-squares mean difference of 0.36 stools/day [95% CI: 0.16 to 0.56]. With respect to secondary outcome variables, stool consistency and ease of stool passage improved more in the PEG 4000 group (p = 0.001). The incidence of adverse events was similar in both groups, the majority of which were mild.
Conclusions: PEG 4000 has superior efficacy to lactulose for the treatment of chronic constipation in young children and is well tolerated.
Trial registration: US National Institute of Health Clinical Trials database; study NCT00255372 first registered 17th November 2005.
Figures
Similar articles
-
Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review).Evid Based Child Health. 2013 Jan;8(1):57-109. doi: 10.1002/ebch.1893. Evid Based Child Health. 2013. PMID: 23878124 Review.
-
PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial.Gut. 2004 Nov;53(11):1590-4. doi: 10.1136/gut.2004.043620. Gut. 2004. PMID: 15479678 Free PMC article. Clinical Trial.
-
Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children.J Pediatr Gastroenterol Nutr. 2005 Nov;41(5):625-33. doi: 10.1097/01.mpg.0000181188.01887.78. J Pediatr Gastroenterol Nutr. 2005. PMID: 16254521 Clinical Trial.
-
Tolerance and Long-Term Efficacy of Polyethylene Glycol 4000 (Forlax®) Compared to Lactulose in Elderly Patients with Chronic Constipation.J Nutr Health Aging. 2017;21(4):429-439. doi: 10.1007/s12603-016-0762-6. J Nutr Health Aging. 2017. PMID: 28346570 Clinical Trial.
-
A literature review of two laxatives: lactulose and polyethylene glycol.Br J Community Nurs. 2011 Dec;16(12):584, 586, 588-90. doi: 10.12968/bjcn.2011.16.12.584. Br J Community Nurs. 2011. PMID: 22413403 Review.
Cited by
-
Effect and cerebral mechanism of acupuncture treatment for functional constipation: study protocol for a randomized controlled clinical trial.Trials. 2019 May 24;20(1):283. doi: 10.1186/s13063-019-3410-8. Trials. 2019. PMID: 31126315 Free PMC article.
-
Tethered cord syndrome (TCS) and constipation in children: a multifaceted approach (literature review).Ann Med Surg (Lond). 2025 Feb 27;87(3):1529-1542. doi: 10.1097/MS9.0000000000003044. eCollection 2025 Mar. Ann Med Surg (Lond). 2025. PMID: 40213204 Free PMC article. Review.
-
Clinical Features of Severely Constipated Children: Comparison of Infrequent Bowel Movement and Fecal Soiling Groups.Pediatr Gastroenterol Hepatol Nutr. 2020 Jan;23(1):26-34. doi: 10.5223/pghn.2020.23.1.26. Epub 2020 Jan 8. Pediatr Gastroenterol Hepatol Nutr. 2020. PMID: 31988873 Free PMC article.
-
Strategies used for childhood chronic functional constipation: the SUCCESS evidence synthesis.Health Technol Assess. 2024 Jan;28(5):1-266. doi: 10.3310/PLTR9622. Health Technol Assess. 2024. PMID: 38343084 Free PMC article.
-
Analysis of the efficacy of lactulose combined with polyethylene glycol in the treatment of functional constipation.Am J Transl Res. 2024 Dec 15;16(12):7491-7500. doi: 10.62347/TJRK1442. eCollection 2024. Am J Transl Res. 2024. PMID: 39822517 Free PMC article.
References
-
- Loening-Baucke V. Chronic constipation in children. Gastroenterology. 1993;105(5):1557–1564. - PubMed
-
- van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101(10):2401–2409. - PubMed
-
- Ip KS, Lee WT, Chan JS, Young BW. A community-based study of the prevalence of constipation in young children and the role of dietary fibre. Hong Kong Med J. 2005;11(6):431–436. - PubMed
-
- Zhou H, Yao M, Cheng GY, Chen YP, Li DG. Prevalence and associated factors of functional gastrointestinal disorders and bowel habits in Chinese adolescents: a school-based study. J Pediatr Gastroenterol Nutr. 2011;53(2):168–173. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical