Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs
- PMID: 24943108
- PMCID: PMC4068960
- DOI: 10.1186/1471-2172-15-24
Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs
Abstract
Background: Immunoregulatory probiotics (immunobiotics) have been proposed to improve piglets' immune system to avoid intestinal infections and reduce unproductive inflammation after weaning. Previously, it was demonstrated that Lactobacillus jensenii TL2937 (LjTL2937) attenuated the inflammatory response triggered by activation of Toll-like receptor 4 (TLR-4) in porcine intestinal epithelial (PIE) cells and antigen presenting cells (APCs) from porcine Peyer's patches (PP).
Objective: In view of the critical importance of PIE-APCs interactions in the regulation of intestinal immune responses, we aimed to examine the effect of LjTL2937 on activation patterns of APCs from swine PPs in co-cultures with PIE cells. In addition, we investigated whether LjTL2937 was able to beneficially modulate intestinal immunity of piglets after weaning to improve immune-health status.
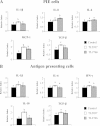
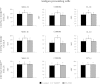
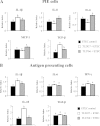
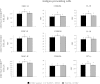
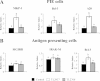
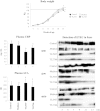
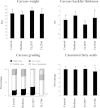
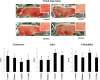
Results: Stimulation of PIE-APCs co-cultures with LjTL2937 increased the expression of MHC-II, CD80/86, IL-10, and Bcl-3 in CD172a+CD11R1- and CD172a+CD11R1high APCs. In addition, the TL2937 strain caused the upregulation of three negative regulators of TLR4 in PIE cells: MKP-1, Bcl-3 and A20. These changes significantly reduced the inflammatory response triggered by TLR4 activation in PIE-APCs co-cultures. The in vivo experiments using castrated male piglets (crossbreeding (LWD) with Landrace (L), Large Yorkshire (W) and Duroc (D))of 3 weeks of age demonstrated that feeding with LjTL2937 significantly reduced blood complement activity and C reactive protein concentrations while no changes were observed in blood leukocytes, ratio of granulocytes to lymphocyte numbers, macrophages' activity and antibody levels. In addition, treatment with LjTL2937 significantly improved growth performance and productivity, and increased carcass quality.
Conclusions: We demonstrated that the use of immunobiotics strains like LjTL2937, as supplemental additives for piglets feedings, could be used as a strategy to maintain and improve intestinal homeostasis; that is important for the development of the pig and for health and performance throughout the productive life of the animal.
Figures

References
-
- Blecha J. In: Biology of Domestic Pigs. Pand WG, Mersmand HJ, editor. Ithaca, NY, USA: Cornell University; 2001. Immunology; pp. 688–711.
-
- McCracken BA, Gaskins HR, Ruwekaiser PJ, Klasing KC, Jewell DE. Diet-dependent and diet-independent metabolic responses underlie growth stasis of pigs at weaning. J Nutr. 1995;125:2838–2845. - PubMed
-
- McCracken BA, Spurlock ME, Roos MA, Zuckermann FA, Gaskins HR. Weaning anorexia may contribute to local inflammation in the pig- let small intestine. J Nutr. 1999;129:613–619. - PubMed
-
- Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134:641–647. - PubMed
-
- Williams BA, Verstegen MWA, Tamminga S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr Res Rev. 2001;14:207–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

