Pilus distribution among lineages of group b streptococcus: an evolutionary and clinical perspective
- PMID: 24943359
- PMCID: PMC4074840
- DOI: 10.1186/1471-2180-14-159
Pilus distribution among lineages of group b streptococcus: an evolutionary and clinical perspective
Abstract
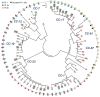
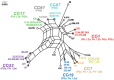
Background: Group B Streptococcus (GBS) is an opportunistic pathogen in both humans and bovines. Epidemiological and phylogenetic analyses have found strains belonging to certain phylogenetic lineages to be more frequently associated with invasive newborn disease, asymptomatic maternal colonization, and subclinical bovine mastitis. Pilus structures in GBS facilitate colonization and invasion of host tissues and play a role in biofilm formation, though few large-scale studies have estimated the frequency and diversity of the three pilus islands (PIs) across diverse genotypes. Here, we examined the distribution of pilus islands (PI) 1, 2a and 2b among 295 GBS strains representing 73 multilocus sequence types (STs) belonging to eight clonal complexes. PCR-based RFLP was also used to evaluate variation in the genes encoding pilus backbone proteins of PI-2a and PI-2b.
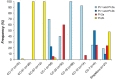
Results: All 295 strains harbored one of the PI-2 variants and most human-derived strains contained PI-1. Bovine-derived strains lacked PI-1 and possessed a unique PI-2b backbone protein allele. Neonatal strains more frequently had PI-1 and a PI-2 variant than maternal colonizing strains, and most CC-17 strains had PI-1 and PI-2b with a distinct backbone protein allele. Furthermore, we present evidence for the frequent gain and loss of genes encoding certain pilus types.
Conclusions: These data suggest that pilus combinations impact host specificity and disease presentation and that diversification often involves the loss or acquisition of PIs. Such findings have implications for the development of GBS vaccines that target the three pilus islands.
Figures


References
-
- Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005;41(6):839–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

