Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations
- PMID: 24943716
- PMCID: PMC4097831
- DOI: 10.1186/scrt468
Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations
Abstract
Introduction: Pathophysiologic changes associated with diabetes impair new blood vessel formation and wound healing. Mesenchymal stem cells derived from adipose tissue (ASCs) have been used clinically to promote healing, although it remains unclear whether diabetes impairs their functional and therapeutic capacity.
Methods: In this study, we examined the impact of diabetes on the murine ASC niche as well as on the potential of isolated cells to promote neovascularization in vitro and in vivo. A novel single-cell analytical approach was used to interrogate ASC heterogeneity and subpopulation dynamics in this pathologic setting.
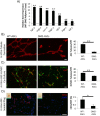
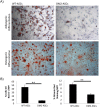
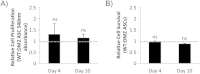
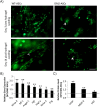
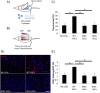
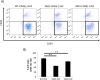
Results: Our results demonstrate that diabetes alters the ASC niche in situ and that diabetic ASCs are compromised in their ability to establish a vascular network both in vitro and in vivo. Moreover, these diabetic cells were ineffective in promoting soft tissue neovascularization and wound healing. Single-cell transcriptional analysis identified a subpopulation of cells which was diminished in both type 1 and type 2 models of diabetes. These cells were characterized by the high expression of genes known to be important for new blood vessel growth.
Conclusions: Perturbations in specific cellular subpopulations, visible only on a single-cell level, represent a previously unreported mechanism for the dysfunction of diabetic ASCs. These data suggest that the utility of autologous ASCs for cell-based therapies in patients with diabetes may be limited and that interventions to improve cell function before application are warranted.
Figures

Comment in
-
Hyperglycemia diverts dividing stem cells to pathological adipogenesis.Stem Cell Res Ther. 2014 Nov 17;5(6):128. doi: 10.1186/scrt518. Stem Cell Res Ther. 2014. PMID: 25689074 Free PMC article.
References
-
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. - PubMed
-
- Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP, Wong VW, Brownlee M, Gurtner GC. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9:75–79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

