Coordination of the filament stabilizing versus destabilizing activities of cofilin through its secondary binding site on actin
- PMID: 24943913
- PMCID: PMC4241054
- DOI: 10.1002/cm.21178
Coordination of the filament stabilizing versus destabilizing activities of cofilin through its secondary binding site on actin
Abstract
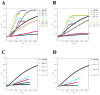
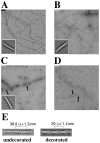
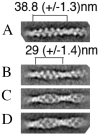
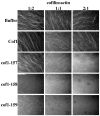
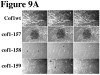
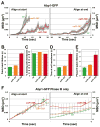
Cofilin is a ubiquitous modulator of actin cytoskeleton dynamics that can both stabilize and destabilize actin filaments depending on its concentration and/or the presence of regulatory co-factors. Three charge-reversal mutants of yeast cofilin, located in cofilin's filament-specific secondary binding site, were characterized in order to understand why disruption of this site leads to enhanced filament disassembly. Crystal structures of the mutants showed that the mutations specifically affect the secondary actin-binding interface, leaving the primary binding site unaltered. The mutant cofilins show enhanced activity compared to wild-type cofilin in severing and disassembling actin filaments. Electron microscopy and image analysis revealed long actin filaments in the presence of wild-type cofilin, while the mutants induced many short filaments, consistent with enhanced severing. Real-time fluorescence microscopy of labeled actin filaments confirmed that the mutants, unlike wild-type cofilin, were functioning as constitutively active severing proteins. In cells, the mutant cofilins delayed endocytosis, which depends on rapid actin turnover. We conclude that mutating cofilin's secondary actin-binding site increases cofilin's ability to sever and de-polymerize actin filaments. We hypothesize that activators of cofilin severing, like Aip1p, may act by disrupting the interface between cofilin's secondary actin-binding site and the actin filament.
Keywords: S. cerevisiae; actin cytoskeleton; cofilin; endocytosis; severing.
© 2014 Wiley Periodicals, Inc.
Figures
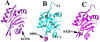
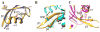




References
-
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. - PubMed
-
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1989.
-
- Balcer HI, Goodman AL, Rodal AA, Smith E, Kugler J, Heuser JE, Goode BL. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr Biol. 2003;13:2159–2169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

