Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial
- PMID: 24944260
- PMCID: PMC4115605
- DOI: 10.1212/WNL.0000000000000615
Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial
Abstract
Objective: To determine whether droxidopa, an oral norepinephrine precursor, improves symptomatic neurogenic orthostatic hypotension (nOH).
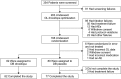
Methods: Patients with symptomatic nOH due to Parkinson disease, multiple system atrophy, pure autonomic failure, or nondiabetic autonomic neuropathy underwent open-label droxidopa dose optimization (100-600 mg 3 times daily), followed, in responders, by 7-day washout and then a 7-day double-blind trial of droxidopa vs placebo. Outcome measures included patient self-ratings on the Orthostatic Hypotension Questionnaire (OHQ), a validated, nOH-specific tool that assesses symptom severity and symptom impact on daily activities.
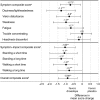
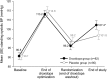
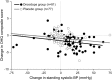
Results: From randomization to endpoint (n = 162), improvement in mean OHQ composite score favored droxidopa over placebo by 0.90 units (p = 0.003). Improvement in OHQ symptom subscore favored droxidopa by 0.73 units (p = 0.010), with maximum change in "dizziness/lightheadedness." Improvement in symptom-impact subscore favored droxidopa by 1.06 units (p = 0.003), with maximum change for "standing a long time." Mean standing systolic blood pressure (BP) increased by 11.2 vs 3.9 mm Hg (p < 0.001), and mean supine systolic BP by 7.6 vs 0.8 mm Hg (p < 0.001). At endpoint, supine systolic BP >180 mm Hg was observed in 4.9% of droxidopa and 2.5% of placebo recipients. Adverse events reported in ≥ 3% of double-blind droxidopa recipients were headache (7.4%) and dizziness (3.7%). No patients discontinued double-blind treatment because of adverse events.
Conclusions: In patients with symptomatic nOH, droxidopa improved symptoms and symptom impact on daily activities, with an associated increase in standing systolic BP, and was generally well tolerated.
Classification of evidence: This study provides Class I evidence that in patients with symptomatic nOH who respond to open-label droxidopa, droxidopa improves subjective and objective manifestation of nOH at 7 days.
© 2014 American Academy of Neurology.
Figures
Similar articles
-
Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa.Hypertension. 2015 Jan;65(1):101-7. doi: 10.1161/HYPERTENSIONAHA.114.04035. Epub 2014 Oct 27. Hypertension. 2015. PMID: 25350981 Free PMC article. Clinical Trial.
-
Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (nOH306B).Mov Disord. 2015 Apr 15;30(5):646-54. doi: 10.1002/mds.26086. Epub 2014 Dec 9. Mov Disord. 2015. PMID: 25487613 Clinical Trial.
-
Long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension.J Am Soc Hypertens. 2016 Oct;10(10):755-762. doi: 10.1016/j.jash.2016.07.010. Epub 2016 Aug 4. J Am Soc Hypertens. 2016. PMID: 27614923 Clinical Trial.
-
Neurogenic orthostatic hypotension in Parkinson's disease: evaluation, management, and emerging role of droxidopa.Vasc Health Risk Manag. 2014 Apr 3;10:169-76. doi: 10.2147/VHRM.S53983. eCollection 2014. Vasc Health Risk Manag. 2014. PMID: 24729712 Free PMC article. Review.
-
Meta-analysis of the safety and efficacy of droxidopa for neurogenic orthostatic hypotension.Clin Auton Res. 2016 Jun;26(3):171-80. doi: 10.1007/s10286-016-0349-7. Epub 2016 Mar 7. Clin Auton Res. 2016. PMID: 26951135 Review.
Cited by
-
Lifetime evolution of ADHD treatment.J Neural Transm (Vienna). 2021 Jul;128(7):1085-1098. doi: 10.1007/s00702-021-02336-w. Epub 2021 May 15. J Neural Transm (Vienna). 2021. PMID: 33993352 Review.
-
Management Strategies for Comorbid Supine Hypertension in Patients with Neurogenic Orthostatic Hypotension.Curr Neurol Neurosci Rep. 2021 Mar 9;21(4):18. doi: 10.1007/s11910-021-01104-3. Curr Neurol Neurosci Rep. 2021. PMID: 33687577 Free PMC article. Review.
-
Multiple system atrophy.Nat Rev Dis Primers. 2022 Aug 25;8(1):56. doi: 10.1038/s41572-022-00382-6. Nat Rev Dis Primers. 2022. PMID: 36008429 Review.
-
Role of nurses and nurse practitioners in the recognition, diagnosis, and management of neurogenic orthostatic hypotension: a narrative review.Int J Gen Med. 2019 May 1;12:173-184. doi: 10.2147/IJGM.S170655. eCollection 2019. Int J Gen Med. 2019. PMID: 31118743 Free PMC article. Review.
-
The physiologic complexity of beat-to-beat blood pressure is associated with age-related alterations in blood pressure regulation.Aging Cell. 2024 Jan;23(1):e13943. doi: 10.1111/acel.13943. Epub 2023 Aug 24. Aging Cell. 2024. PMID: 37615223 Free PMC article.
References
-
- Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996;6:125–126 - PubMed
-
- Freeman R. Clinical practice: neurogenic orthostatic hypotension. N Engl J Med 2008;358:615–624 - PubMed
-
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72 - PubMed
-
- Mann S, Altman DG, Raftery EB, Bannister R. Circadian variation of blood pressure in autonomic failure. Circulation 1983;68:477–483 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
