Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation
- PMID: 24944268
- PMCID: PMC7199227
- DOI: 10.1152/ajprenal.00127.2014
Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation
Abstract
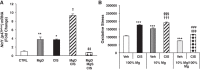
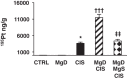
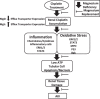
Despite its success as a potent antineoplastic agent, ∼25% of patients receiving cisplatin experience acute kidney injury (AKI) and must discontinue therapy. Impaired magnesium homeostasis has been linked to cisplatin-mediated AKI, and because magnesium deficiency is widespread, we examined the effect of magnesium deficiency and replacement on cisplatin-induced AKI in physiologically relevant older female mice. Magnesium deficiency significantly increased cisplatin-associated weight loss and markers of renal damage (plasma blood urea nitrogen and creatinine), histological changes, inflammation, and renal cell apoptosis and modulated signaling pathways (e.g., ERK1/2, p53, and STAT3). Conversely, these damaging effects were reversed by magnesium. Magnesium deficiency alone significantly induced basal and cisplatin-mediated oxidative stress, whereas magnesium replacement attenuated these effects. Similar results were observed using cisplatin-treated LLC-PK1 renal epithelial cells exposed to various magnesium concentrations. Magnesium deficiency significantly amplified renal platinum accumulation, whereas magnesium replacement blocked the augmented platinum accumulation after magnesium deficiency. Increased renal platinum accumulation during magnesium deficiency was accompanied by reduced renal efflux transporter expression, which was reversed by magnesium replacement. These findings demonstrate the role of magnesium in regulating cisplatin-induced AKI by enhancing oxidative stress and thus promoting cisplatin-mediated damage. Additional in vitro experiments using ovarian, breast, and lung cancer cell lines showed that magnesium supplementation did not compromise cisplatin's chemotherapeutic efficacy. Finally, because no consistently successful therapy to prevent or treat cisplatin-mediated AKI is available for humans, these results support developing more conservative magnesium replacement guidelines for reducing cisplatin-induced AKI in cancer patients at risk for magnesium deficiency.
Keywords: apoptosis; hypomagnesemia; inflammation; nephrotoxicity; oxidative stress.
Copyright © 2014 the American Physiological Society.
Figures









References
-
- Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O'Hare AM, Schmader KE, High KP. Acute kidney injury in older adults. J Am Soc Nephrol : 28–38, 2011. - PubMed
-
- Aoki M, Terada T, Kajiwara M, Ogasawara K, Ikai I, Ogawa O, Katsura T, Inui K. Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Renal Physiol : F165–F170, 2008. - PubMed
-
- Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr Suppl : S24–S36, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

