Bayesian pharmacokinetic analysis of a gentamicin nomogram in neonates: a retrospective study
- PMID: 24944364
- PMCID: PMC4052991
- DOI: 10.1016/S0011-393X(03)00022-5
Bayesian pharmacokinetic analysis of a gentamicin nomogram in neonates: a retrospective study
Abstract
Background: Although gentamicin is used extensively within the first week of life for suspected sepsis in neonates, little is known about the performance of gentamicin dosing nomograms in this population.
Objective: The goal of our study was to retrospectively assess the performance of a gentamicin dosing nomogram in neonates given gentamicin during the first week after birth.
Methods: In this retrospective study, gentamicin therapeutic drug monitoring data were collected during routine clinical care for all neonates who were born in St. Boniface General Hospital (Winnipeg, Manitoba, Canada) between January 1999 and April 2001 and given gentamicin during the first week after birth. We used Bayesian pharmacokinetic analysis to retrospectively assess the performance of our gentamicin dosing nomogram in neonates born at gestation ages <32 weeks, between 32 and 34 weeks, and >34 weeks. Bayesian pharmacokinetic values for parameters within groups were compared and used to explore predicted peak and trough serum gentamicin concentrations based on the institutional dosing nomogram.
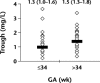
Results: In a total of 58 neonates, those neonates born at ≤34 weeks' gestation had a weight-normalized apparent volume of gentamicin distribution 1.6 times larger than infants born after 34 weeks' gestation (P<0.001), as identified by Bayesian analysis. Weight-normalized gentamicin clearance was 22% lower in the youngest age category (P<0.01). Only 33% of predicted peak serum gentamicin concentrations were >6 mg/L for neonates born at ≤34 weeks' gestation, whereas 90% were therapeutic in neonates born at >34 weeks' gestation (P<0.001). With the present nomogram, the likelihood of an indication for adjustment of the dosing regimen was 12.4-fold higher (95% CI, 3.5-43.7) for those neonates born at ≤34 weeks' gestation.
Conclusions: These results have important clinical implications with regard to the advisability of determining peak serum gentamicin concentrations in neonates born at ≤34 weeks' gestation. Sampling of peak serum concentrations is indicated in this population to avoid underdosing and potential loss of therapeutic efficacy.
Keywords: Bayesian pharmacokinetics; gentamicin; neonatal sepsis; nomograms.
Figures



References
-
- Triggs E., Charles B. Pharmacokinetics and therapeutic drug monitoring of gentamicin in the elderly. Clin Pharmacokinet. 1999;37:331–341. - PubMed
-
- Rajchgot P., Prober C.G., Soldin S. Aminoglycoside-related nephrotoxicity in the premature newborn. Clin Pharmacol Ther. 1984;35:394–401. - PubMed
-
- McCracken G.H., Jr. Aminoglycoside toxicity in infants and children. Am J Med. 1986;80:172–178. - PubMed
-
- Hauch A.M., Peitersen B., Peitersen E. Vestibular toxicity following netilmicin therapy in the neonatal period. Dan Med Bull. 1986;33:107–109. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

